Research Article - Modern Phytomorphology ( 2025) Volume 19, Issue 3
The anti-inflammatory effect of Artemisia absinthium extract on cell growth through targeting PI3K/Akt/mTOR pathway in human hepatocellular carcinoma cell line
Nuha A. Alkhattabi* and Nesrin I. Tarbiah*Nuha A. Alkhattabi, Biochemistry Department, Faculty of Science, King Abdulaziz University, Jeddah 21589, Saudi Arabia, Email: ntarabah@kau.edu.sa Nesrin I. Tarbiah, Department of Biochemistry, King Abdulaziz University, Jeddah, Saudi Arabia, Email: ntarabah@kau.edu.sa
Received: 18-Jul-2025, Manuscript No. mp-25-168034; , Pre QC No. mp-25-168034 (PQ); Editor assigned: 21-Jul-2025, Pre QC No. mp-25-168034 (PQ); Reviewed: 04-Aug-2025, QC No. mp-25-168034; Revised: 11-Aug-2025, Manuscript No. mp-25-168034 (R); Published: 18-Aug-2025, DOI: 10.5281/zenodo.16809969
Abstract
Objective: Artemisia absinthium’s biological activity has been investigated for possible therapeutic applications. It is a plant that may be used in cancer treatment. The biochemical and molecular mechanisms of Artemisia absinthium Extract (AAE) treatment in the hepatic cancer HepG-2 cell line were investigated in this work.
Method: Phytochemical profiling was performed using quantitative methods and GC-MS analysis to assess phytoconstituents in AAE. The in vitro cytotoxic effect of AAE on HepG-2 cellular viability was determined to assess the inhibitory concentrations that kill 50% of cells (IC50, μg/ml). Apoptosis and cell cycle arrest were investigated using flow cytometry analysis. Furthermore, gene expression levels of PI3K/Akt/mTOR genes as well as their protein levels in the AAE-treated HepG-2 and untreated cells, were evaluated.
Results: The results showed that AAE has adequate phytochemical content. The IC50 of AAE for HepG-2cells was calculated after 48 hours to be 186.89 ± 1.56 μg/ml. AAE treatment led to a marked decrease in the percentages of viable HepG-2 cells with a significant increase in the percentages of necrotic and apoptotic cells. Significant increase in the HepG-2 count was recorded in the sub G1 and G1 phases post the treatment with AAE IC50. The treatment with AAE led to a significant decrease in the cells’ proportion in the S-phase and G2/M phase. AAE treatment resulted in significant downregulation in the expression of the PI3K, Akt, and mTOR genes and their protein in HepG-2 cell lines.
Conclusion: Targeting the PI3K/Akt/mTOR signaling pathway, the results suggest that AAE has the potential to be an anti-inflammatory and anti-proliferative agent against HepG-2 cells.
Keywords
Artemisia absinthium, Hepatocellular carcinoma, Apoptosis, Cell cycle, Signal pathway
Introduction
Because of its complexity, cancer research has always been extremely challenging. Different cancers can differ significantly in their prognosis, organ effects, genetic changes, and treatment modalities (Huang and Zhang, 2022). The most common cancerous tumor that cause death worldwide is Hepatocellular Carcinoma (HCC) (Bray, et al. 2024). Infections, alcoholism, tobacco use, and obesity are the main risk factors for Hepatocellular Carcinoma (HCC) (London, et al. 2018). Currently, liver transplantation, chemotherapy drugs, and immunotherapeutic approaches are used to treat HCC (Luo, et al. 2022). Oxaliplatin and sorafenib, the two main pharmaceutical treatments for hepatocellular carcinoma, are still insufficient because of their side effects and the emergence of drug resistance. As a result, finding a novel therapeutic agents to treat HCC is crucial (Wei, et al. 2019).
Growing interest in using medicinal plants in developing nations is due to the belief that herbal medicines are safe and have few to no side effects, especially when compared to modern pharmaceuticals (Shaikh, et al. 2016). Herbal medicines contain natural ingredients that may have anti-cancer effects due to their varied chemical compositions and bioactivity (JenÄa, et al. 2024). There are several ways in which these herbal remedies and their bioactive components can successfully treat HCC. Certain natural herbal extracts have also been shown to be effective in the treatment of HCC (Abdel-Hamid, et al. 2018 and Guo, et al. 2024). A number of plant derivatives have become essential tools in oncological research and treatment development because of their pleiotropic properties, which include scavenging free radicals, inhibiting cell growth, and inducing apoptosis (Rawat, et al. 2018).
Potential anticancer drugs that mostly induce death to tumor cells and rarely damage healthy cells have been studied. hese phytochemicals have the potential to cause apoptosis, stop tumor cell invasion and migration, and suppress angiogenesis and proliferation (Wang, et al. 2023). Additionally, research has demonstrated the anti-carcinogenic qualities of a number of compounds derived from plant parts, such as seeds, fruits, bark, roots, and leaves. By inducing apoptosis and inhibiting the oxidative stress/inflammatory pathway, herbal extracts improved hepatocellular carcinoma (Asma, et al. 2022). Herbs from the Artemisia plant are used ethnopharmacologically and traditionally to treat a variety of illnesses. Wormwood, or Artemisia absinthium L., is a well-known plant in the Asteraceae family with significant therapeutic and commercial uses. It contains a variety of bioactive chemical components that have antimicrobial, hepatoprotective, anti-inflammatory, and antioxidant qualities; these compounds may be used in medicine (Sharifi-Rad, et al. 2022 and Wubuli, et al. 2024). By causing endoplasmic reticulum stress and apoptosis through a mitochondrial-dependent mechanism, the extract from A. absinthium L. suppressed the growth of hepatocellular carcinoma cells (Wei, et al. 2019). In addition, reducing inflammation and oxidative stress, Artemisia annua extract reduced liver damage (El-Said, et al. 2023). Focusing on the Wnt/β-Catenin pathway, Artemisia argyi suppresses hepatocellular cancer (Li, et al. 2021). Malhab, et al. examined the anticancer potential of Artemisia herba-alba using colorectal cancer cell lines (Mlhab, et al. 2024), while Kim, et al. discovered that Artemisia capillaris significantly inhibited cell proliferation, promoted apoptosis, and decreased the PI3K/AKT pathway in Hepatocellular Carcinoma (HCC) (Kim, et al. 2018). Moreover, Tsamesidis, et al. investigated the cytotoxic effects of A. absinthium extract on an oral carcinoma cell line in 2024 (Tsamesidis, et al. 2024). The plant Artemisia has been shown to have anti-inflammatory, anti-fibrotic, antioxidant, and anti-cancer qualities (Jang, et al. 2015).
To prevent and treat liver cancer, a novel drug with high efficacy and minimal side effects is desperately needed. This study used the HepG-2 cell line, a model of human hepatic cancer, to investigate the molecular and biochemical mechanisms underlying AAE’s anticancer effects.
Materials and Methods
Preparation of plant materials
A. absinthium were brought from Carrefour Market. The plant met the institutional requirements and was verified by an expert. The seeds were ground into a powder, then fifty grams of the powder in 500 mL of ethanol (70%) were filtered, and A. absinthium Extract (AAE) was obtained.
Phytochemicals analysis and GC-MS profiling
The AAE was used to assess the phytochemicals’ capacity to scavenge DPPH radicals (Blios, 1958, Hiai, et al. 1975, Singleton, et al. 1999 and Prieto, et al. 1999). The phytochemicals in the AAE were identified using a mass spectrometer (GC 1310-ISQ, Thermo Scientific, Austin, TX, USA). The components were identified, and their retention periods and mass spectra were compared to those in the NIST 11 and WILEY 09 mass spectra databases.
Cancer cell line and treatment protocols
The American Type Culture Collection (ATCC) provided the HepG-2, which was cultivated at 37°C in 95% air and 5% CO2 with 10% FBS added in Dulbecco’s Modified Eagle’s Medium (DMEM) that was not heat-inactivated. Cells will be cultivated for 24 hours in a medium containing 2% FBS before each experiment that helps cells adhere.
Cell viability
In a 96-well plate, HepG-2 cells were planted in triplicate (1 × 104 cells/well). After 48 hours of treatment with varying doses of AAE (0, 10, 20, 40, 80, and 100 μg/ml) or control, 20 μL of MTT reagent was applied to each well, and the cells were incubated for two hours to allow for formazan crystallization. Using a Spectra Max M5 spectrophotometer, absorbance at 570 nm was measured. The half inhibitory effective concentration (IC50) was evaluated and used for further studies.
Flow cytometry analysis
Apoptosis was assessed using a flow cytometer (Becton Dickinson, Sunnyvale, CA, USA) and an apoptosis kit (catalog no. V13242). The percentages of cells were computed and the flow cytometry histogram was generated. Furthermore, the cell cycle study’s findings supported those of Weir, et al. After being fixed and stained with Dye Cycle Violet stain (catalog no. V35003), the cells were examined using flow cytometry (Weir, et al. 2007).
Biochemical analysis
Levels of IL-6 (Cat. no. MBS021993), IL-1β (Cat. no. MBS263843), TNF-α (Cat. no. MBS2502004), NF-κB (Cat. no. MBS260718), MDA (cat. no. MBS263626), SOD (cat. no. MBS2022511), CAT (cat. no. MBS2021346), and GSH (cat. no. MBS727656) were determined in the cell’s lysate using their ELISA kits. Furthermore, the p-PI3K (Cat. no. MBS9518759), p-Akt (Cat. no. MBS9518725), and p-mTOR (Cat. no. MBS260120) were determined in HepG-2 cells before and after AAE treatment using their ELISA kits from MyBioSource, San Diego, CA, USA.
Gene expression analysis
Using SYBR Green, the HepG-2 cells’ expression of the PI3K, Akt, and mTOR genes were assessed both before and after the AAE treatment (Livak, et al. 2001). The NCBI Primer-Blast tool was used to produce gene-specific primers post β-actin housekeeping gene normalization (Tab. 1).
| Gene | Accession number | Forward sequence (5′-3′) | Reverse sequence (5′-3′) |
|---|---|---|---|
| PI3K | NM_001024955 | CTCTCCTGTGCTGGCTACTGT | GCTCTCGGTTGATTCCAAACT |
| Akt | NM_001165894 | ATCCCCTCAACAACTTCTCAGT | CTTCCGTCCACTCTTCTCTTTC |
| mTOR | NM_020009 | AGAAGGGTCTCCAAGGACGACT | GCAGGACACAAAGGCAGCATTG |
| β-actin | NM_007393 | CCTGTATGCCTCTGGTCGTA | CCACATAGCACAACTTCTCCTT |
| PI3K: Phosphoinositide 3-Kinase; Akt: serine/threonine kinase; mTOR: mammalian Target Of Rapamycin | |||
Table 1. Forward and reverse primer sequences for RT-PCR.
Statistical analysis
To assess the one-way ANOVA data, the Graph Pad Prism software (San Diego, CA) (https://www.graphpad.com/) was used. A significance level of P<0.05 was considered acceptable.
Results
Phytochemical constitutes Artemisia absinthium
After being cleaned with tap water and allowed to dry in the shade, A. absinthium (AA) was precisely 70% ethanol soaking for three days in a hydro-ethanol solution. According to the findings, AAE had flavonoid and phenolic levels of 21.18 ± 1.95 mg QE/g DW and 39.87 ± 2.69 mg GAE/g DW, respectively. The extract’s level of saponin was 389 ± 4.96 mg/g DW, and its TAC level was 72.65 ± 3.86 mg AAE/g DW. The extract’s ability to remove 50% of DPPH was 5.46 ± 0.85 mg/mL, and its DPPH scavenging activity (%) was 80% ± 3.34 (Tab. 2).
| Phytochemicals | AA |
|---|---|
| Total phenolics (mg GAE/g DW) | 39.87 ± 2.69 |
| Total flavonoids (mg QE/g DW) | 21.18 ± 1.95 |
| Total antioxidant capacity (TAC) (mg AAE/g DW) | 72.65 ± 3.86 |
| Saponin (mg/g DW) | 389 ± 4.96 |
| DPPH scavenging activity (%) | 80 % ± 3.34 |
| IC50 of DPPH (mg/ml) | 5.46 ± 0.85 |
| Note: AA: Artemisia absinthium; GAE: Gallic Acid Equivalent; QE: Quercetin Equivalents; TAC: Total Antioxidant Capacity; AAE: Ascorbic Acid Equivalent; DW: Dry Weight; IC50: Inhibitory Concentration of 50%; DPPH: Diphenyl-1-Picrylhydrazyl | |
Table 2. Phytochemicals analysis of Artemisia absinthium (AA).
Gas-chromatography mass spectrometry analysis of AAE
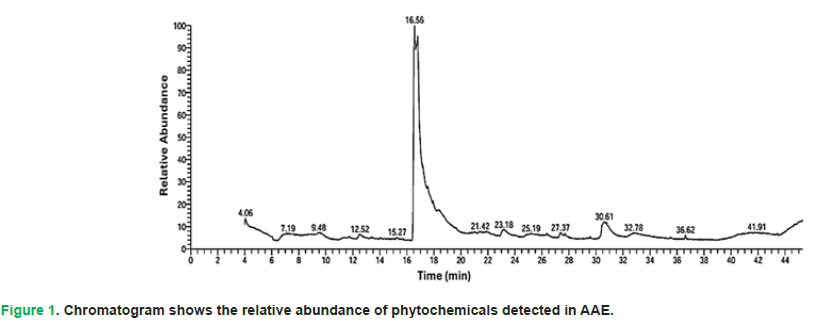
The results showed that the most abundant chemical compounds detected in AAE were 1H-indole-3-ethanamine, N,N-dimethyl, Etilefrine, 9,12-Octadecadienoyl chloride, (Z,Z), Aspidospermidin-17-ol, 1-acetyl-16-methoxy, and benzene methanol, 4-(1-methylethyl). These phytochemicals were recorded at the Retention Times (RTs) 16.55, 16.77, 30.60, 30.79, and 12.51, respectively. The peak areas percentages (P.A %) were 49.86, 27.68, 3.87, 2.04, and 1.33, respectively (Tab. 3 and Fig. 1).
| S. No | R.T (min) | Name | M.W | M.F | P.A (%) |
|---|---|---|---|---|---|
| 1 | 12.51 | Benzene methanol, 4-(1-methylethyl) | 150 | C10H14O | 1.33 |
| 2 | 16.55 | 1H-indole-3-ethanamine, N,N-dimethyl | 907 | C10H15NO | 49.86 |
| 3 | 16.77 | Etilefrine | 828 | C10H15NO2 | 27.68 |
| 4 | 23.19 | 3-Hydroxy-N-methylphenethylamine | 819 | C9H13NO | 1.93 |
| 5 | 27.38 | 1,25-Dihydroxyvitamin D3, TMS derivative | 716 | C30H52O3Si | 1.3 |
| 6 | 30.6 | 9,12-Octadecadienoyl chloride, (Z,Z) | 759 | C18H31ClO | 3.87 |
| 7 | 30.79 | Aspidospermidin-17-ol, 1-acetyl-16-methoxy | 789 | C22H30N2O3 | 2.04 |
| 8 | 32.78 | 2,5-piperazinedione, 3-(4-hydroxyphenyl)methyl-6-methyl | 296.5 | C12H14N2O3 | 1.09 |
| Note: RT: Retention Time; MW: Molecular Weight; MF: Molecular Formula; P.A%: Peak Area Percentage | |||||
Table 3. GC-MS analysis of Artemisia absinthium Extract (AAE).
Figure 1: Chromatogram shows the relative abundance of phytochemicals detected in AAE.
Cytotoxic effect of AAE treatment on HepG-2 cell line
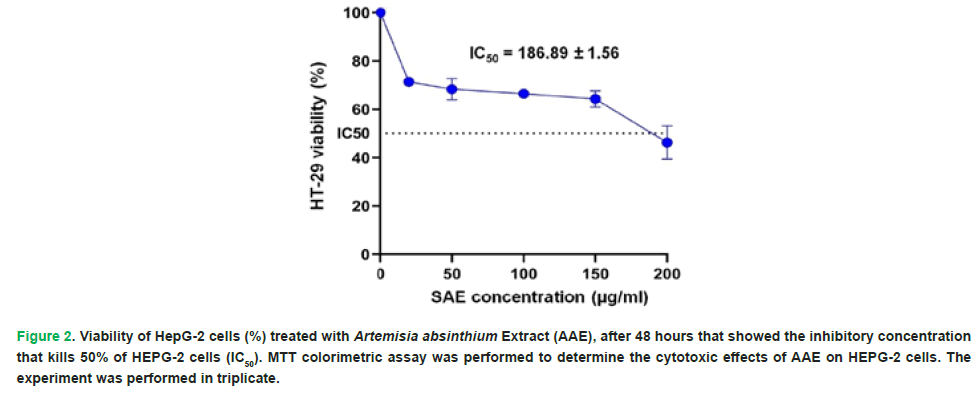
The hydro-ethanolic extract of Artemisia absinthium showed potential anticancer action towards the HepG-2, according to the results of the MTT experiment. After 48 hours, the AAE IC50 for HepG-2 cells was determined to be 186.89 ± 1.56 μg/ml (Fig. 2).
Figure 2: Viability of HepG-2 cells (%) treated with Artemisia absinthium Extract (AAE), after 48 hours that showed the inhibitory concentration that kills 50% of HEPG-2 cells (IC50). MTT colorimetric assay was performed to determine the cytotoxic effects of AAE on HEPG-2 cells. The experiment was performed in triplicate.
Effects of AAE treatment on the percentages of necrotic and apoptotic HepG-2 cells
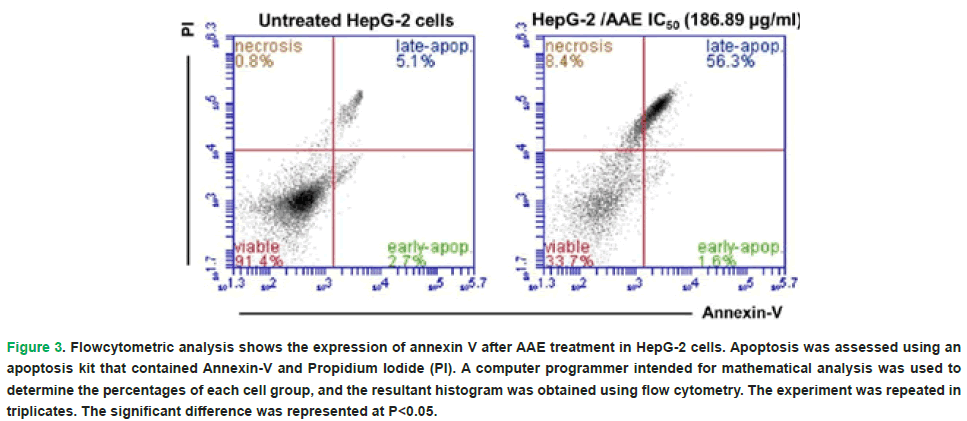
The distribution of HepG-2 cells according to their Annexin/PI staining was changed post the treatment with the IC50 of AAE. Treatment with AAE led to a significant decrease (P<0.05) in the percentage of viable hepatocellular carcinoma cells to 33.7% compared to the untreated HepG-2 (91.4%). However, there is a significant increase in the necrotic HepG-2 cells post AAE treatment to 8.4% compared to the untreated HepG-2 (0.9%). Furthermore, the treatment of HepG-2 cells with the estimated IC50 of AAE showed significant increase (P<0.05) in the late apoptotic cells (%) when compared to the control HepG-2 cells (56.3% versus 5.1%) (Fig. 3).
Figure 3: Flowcytometric analysis shows the expression of annexin V after AAE treatment in HepG-2 cells. Apoptosis was assessed using an apoptosis kit that contained Annexin-V and Propidium Iodide (PI). A computer programmer intended for mathematical analysis was used to determine the percentages of each cell group, and the resultant histogram was obtained using flow cytometry. The experiment was repeated in triplicates. The significant difference was represented at P<0.05.
Effects of AAE treatment on the cell cycle analysis of HepG-2 cells
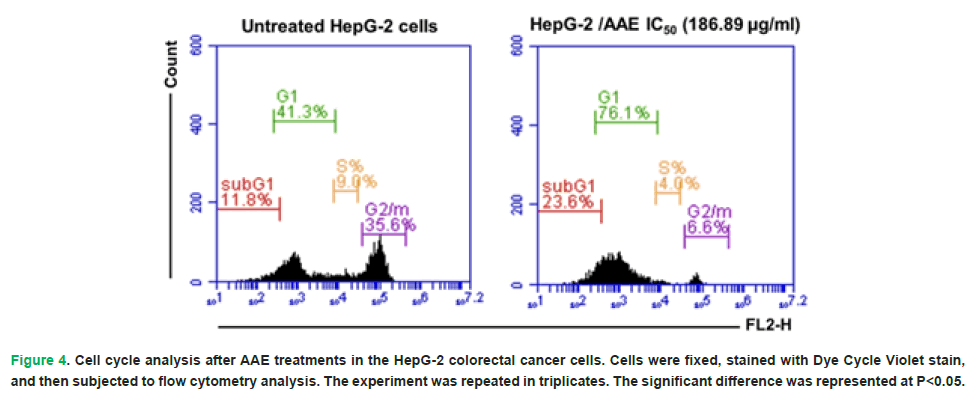
The results represented a significant increase (P<0.05) in the count of HepG-2 cells in the G0/1 (sub G1) phase post the treatment with AAE IC50 to 23.6% when compared to the untreated HepG-2 cells (11.8%). The percentages of HepG-2 cells’ number in the G1-phase increased post the treatment with AAE as compared to control cells (76.1% versus 41.3%). However, the treatment with AAE led to a significant decrease (P<0.05) in the cells’ proportion in the S-phase to 4.0% when compared to the untreated HepG-2 (9.0%). A significant decrease (P<0.05) in the G2/M phase was recorded following the treatment of HepG-2 with AAE to 6.6% when compared to the control cells (35.6%) (Fig. 4).
Figure 4: Cell cycle analysis after AAE treatments in the HepG-2 colorectal cancer cells. Cells were fixed, stained with Dye Cycle Violet stain, and then subjected to flow cytometry analysis. The experiment was repeated in triplicates. The significant difference was represented at P<0.05.
Effects of AAE treatment on inflammatory biomarkers in HepG-2 cells
The results demonstrated a significant decrease (P<0.05) in the levels of IL-6, IL-1β, TNF-α, and NF-κB biomarkers in the AAE-treated HepG-2 cells (15.88 ± 1.31 pg/ml, 13.05 ± 1.12 pg/ml, 22.65 ± 1.57 pg/ml, 1.54 ± 0.17 ng/ml, respectively) when compared to the untreated HepG-2 cells (23.59 ± 1.18 pg/ml, 17.32 ± 1.78 pg/ml, 31.18 ± 1.78 pg/ml, 2.35 ± 0.21 ng/ml, respectively) (Tab. 4).
| Treatment/proteins | IL-6 (pg/mL) | IL-1β (pg/mL) | TNF-α (pg/mL) | NF-κB (ng/mL) |
|---|---|---|---|---|
| Untreated HepG-2 cells | 23.59 ± 1.18 b | 19.32 ±1.78 c | 31.18 ± 1.78 a | 2.35 ± 0.21 a |
| HepG-2 cells/AAE | 15.88 ± 1.31 a | 13.05 ± 1.12 d | 22.65 ± 1.57 b | 1.54 ± 0.17 e |
| Note: AAE: Artemisia absinthium Extract; IL-6: Interleukin 6; IL-1β: Interleukin 1 beta, TNF-α: Tumor Necrosis Factor alpha; NF-κB: Nuclear Factor kappa-B. The experiment was repeated in triplicates. Means that do not share a letter in each column showed significant difference (P<0.05). | ||||
Table 4. Levels of IL-6, IL-1β, TNF-α, and NF-κB in the untreated HepG-2 cells and AAE-treated HepG-2 cells.
Effects of AAE treatment on antioxidant/oxidants’ hemostasis in HepG-2 cells
As shown in Tab. 5, the MDA level was significantly decreased (P<0.05) in the HepG-2 cells that were treated with the IC50 of AAE (1.73 ± 0.12 nmol/mL) when compared to the untreated cells (2.39 ± 0.18 nmol/mL). However, the levels of SOD, CAT, and GSH were significantly increased (P<0.05) after the treatment of HepG-2 cells with AAE to 5.69 ± 0.32, 12.47 ± 1.07, and 16.19 ± 1.79 ng/mL, respectively when compared to the untreated HepG-2 cells (3.57 ± 0.21, 8.32 ± 0.78, and 11.86 ± 1.15 ng/mL, respectively) (Tab. 5).
| Treatment/proteins | MDA (nmol/mL) | SOD (ng/mL) | CAT (ng/mL) | GSH (ng/mL) |
|---|---|---|---|---|
| Untreated HepG-2 cells | 2.39 ± 0.18 a | 3.57 ± 0.21 b | 8.32 ± 0.78 a | 11.86 ± 1.15 c |
| HepG-2 cells/AAE | 1.73 ± 0.12 c | 5.69 ± 0.32 e | 12.47 ± 1.07 f | 16.39 ± 1.79 d |
Note: AAE: Artemisia absinthium extract; MDA: Malondialdehyde; SOD: Superoxide Dismutase; CAT: Catalase; GSH: Reduced Glutathione. The experiment was repeated in triplicates. Means that do not share a letter in each column showed significant difference (P<0.05).
Table 5. Levels of MDA, SOD, CAT, and GSH in the untreated HepG-2 cells and AAE-treated HepG-2 cells.
Effects of AAE treatment on PI3K/Akt/mTOR pathways in HepG-2 cells
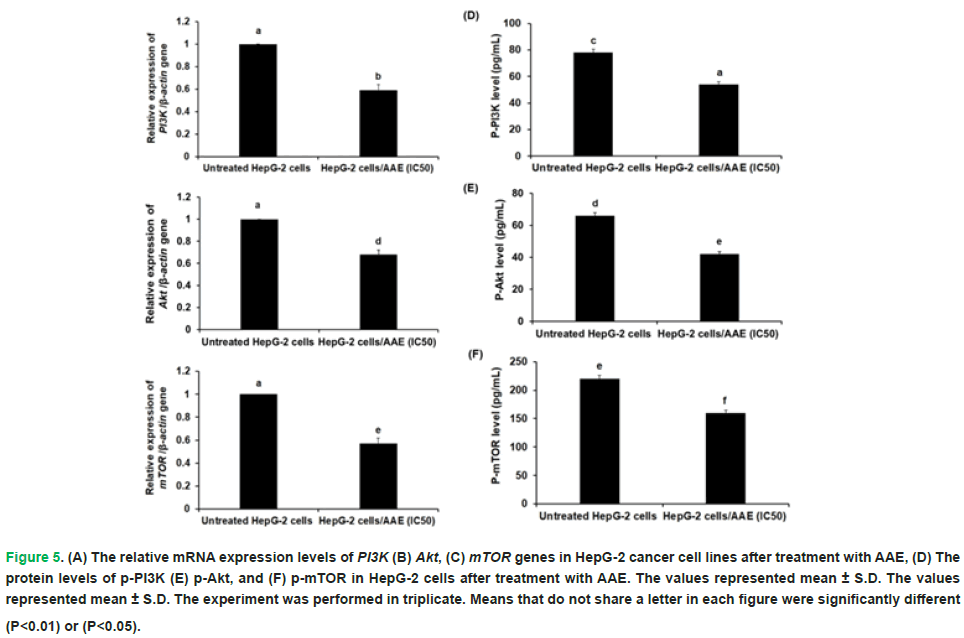
Prior to and following AAE treatment, the human hepatocellular carcinoma cell lines’ expression levels of the PI3K, Akt, and mTOR genes were measured. The findings demonstrated that after adjusting the beta-actin gene by 1.7, 1.5, and 1.8 times, respectively, the expression of the PI3K, Akt, and mTOR genes was considerably downregulated (P<0.01) in HepG-2 cells treated with AAE (Fig. 5 A-C). Additionally, when HepG-2 cells were treated with AAE, their phosphorylation of PI3K and Akt at Tyr607 and Ser473, respectively, was considerably lower than that of the untreated cells (Fig. 5 D-F).
Figure 5: (A) The relative mRNA expression levels of PI3K (B) Akt, (C) mTOR genes in HepG-2 cancer cell lines after treatment with AAE, (D) The protein levels of p-PI3K (E) p-Akt, and (F) p-mTOR in HepG-2 cells after treatment with AAE. The values represented mean ± S.D. The values represented mean ± S.D. The experiment was performed in triplicate. Means that do not share a letter in each figure were significantly different (P<0.01) or (P<0.05).
Discussion
The prognosis for most patients with HCC is still poor, even with the availability of several treatment options, such as chemotherapy (Liu, et al. 2016). Because of its bioactive components, antiproliferative qualities, and anti-inflammatory effects, Artemisia has attracted interest in the treatment of cancer. In Saudi Arabia, wormwood, or A. absinthium as it is scientifically known, is a common ornamental plant. Historically, traditional medicine has relied heavily on this significant perennial shrub (Sultan, et al. 2020). Despite ongoing changes to treatment protocols intended to improve patient survival rates, conventional chemotherapy causes significant side effects because it affects normal cells indiscriminately. Furthermore, the development of novel anticancer compounds with superior pharmacological characteristics is crucial due to the suboptimal pharmacokinetic properties of antineoplastic drugs (Munteanu, et al. 2024). This study evaluated the molecular and biochemical mechanisms of AAE treatment in human HepG-2 cells. Phenolic, flavonoid, and saponin compounds are present in significant amounts in the AAE. The extract concentration needed to reduce 50% of DPPH was 5.46 ± 0.85 mg/mL, and the DPPH scavenging activity was 80% ± 3.34. Furthermore, the findings showed that this extract has many bioactive phytochemicals. The bioactive components of AAE were assessed using GC-MS in a previous study (Renugadevi and Julius, 2020). It has been reported that a wide variety of Artemisia species are cytotoxic to different cell lines (Emami, et al. 2009). The AAE showed possible anticancer activity against the hepatocellular carcinoma cell line and was evaluated as an antiproliferative medication against HepG-2 cells. After 48 hours, the IC50 of AAE for HepG-2 cells was found to be 186.89 ± 1.56 μg/mL. This is consistent with a previous study about A. vulgaris’s ability to inhibit cancer in HepG-2 cells (Sharmila and Padma, 2013). Additionally, Artemisia eriantha has demonstrated possible in vitro effects on hepatocarcinoma cells (Pace, et al. 2024). Chemotherapy and other modern treatment approaches for most cancers entail stimulating the cancer cells’ cellular apoptotic signalling pathways. In order to control carcinogenesis and therapeutic responses, apoptosis is essential. When compared to the untreated HepG-2 cells, treatment with the IC50 dose of AAE significantly increased the number of apoptotic cells. The disruption of the cell cycle, which controls cellular proliferation, is a crucial stage in the development of cancer (Williams and Stoeber, 2012). Therefore, using cell cycle arrest to control cell cycle progression could be a useful cancer treatment strategy. Cell cycle analysis was performed to determine the mechanisms underlying AAE’s effects on hepatocellular carcinoma cell proliferation. The results showed that, after AAE treatment, a higher proportion of HepG-2 cells were in the G1 phase than control cells. Additionally, the fraction of cells in the S-phase increased after AAE was administered. Cell cycle arrest and apoptosis activation were the mechanisms by which the extract from A. judaica demonstrated anticancer efficacy against HepG-2 cells [35]. Previous studies showed that herbal extracts affected HepG-2 cells through the pathways of cell cycle arrest and apoptosis (Wei, et al. 2019, Kim, et al. 2017 and Kim, et al. 2023).
Oxidative stress and chronic inflammation are risk factors for the development of cancer (Sorriento, 2024). The current study found that after HepG-2 cells were treated with AAE, there was a significant decrease in the levels of inflammatory biomarkers. The reduction of inflammatory mediators demonstrated Artemisia capillaris’ anti-inflammatory qualities (Ali, et al. 2021). HepG-2 cells treated with the IC50 of AAE showed a significant decrease in MDA levels. However, after HepG-2 cells were treated with AAE, the levels of antioxidant markers were noticeably higher. These results are consistent with earlier research that described Artemisia’s traits (Abate, et al. 2021, Lee, et al. 2024, Èicolea, et al. 2024, Èicolea, et al. 2025 and Cherfi, et al. 2025).
Natural substances that target the PI3K/Akt and mTOR pathways in hepatocellular cancer have been shown to have therapeutic potential (Sun, et al. 2021, Chen, et al. 2024). In HepG-2 cells treated with AAE, there was a significant downregulation of the expression levels of the PI3K, Akt, and mTOR genes. Additionally, the evaluation of AAE treatment on HepG-2 cells showed a notable decrease in PI3K, Akt, and mTOR protein phosphorylation. Consistent with previous studies, the data show that the PI3K/Akt/mTOR pathway contributes to the improvement of AAE in hepatocellular carcinoma (Sun, et al. 2021, Paskeh, et al. 2023).
Conclusion
According to this study, AAEâs higher phytochemical content causes it to act as a cytotoxic agent against HepG-2 cells. The percentage of apoptotic HepG-2 cells increased significantly because of the AAE treatment, while the S-phase and G2/M phase significantly decreased. After receiving AAE therapy, HepG-2 cells showed significant downregulation of the PI3K, Akt, and mTOR genes as well as their protein levels. These findings show that AAE targets the PI3K/Akt/mTOR pathway to have antiproliferative and anti-inflammatory effects on the HepG-2 cell line.Acknowledgment
The authors gratefully acknowledge continuous support from King Abdulaziz University, Jeddah, Saudi Arabia.Conflict of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.Funding
Not applicable.
References
- Huang B, Zhang Y. (2022). Teaching an old dog new tricks: Drug discovery by repositioning natural products and their derivatives. Drug Discov Today. 27:1936-44.
[Crossref] [Google Scholar] [PubMed]
- Bray F, Laversanne M, Sung H, Ferlay J, Siegel RL, Soerjomataram I, Jemal A. (2024). Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 74:229-63.
[Crossref] [Google Scholar] [PubMed]
- London WT, Petrick JL, McGlynn KA, Thun MJ, Linet MS, Cerhan JR, Haiman CA, Schottenfeld D, (Eds.). (2018) Cancer epidemiology and prevention, 4th ed.; Oxford University Press: New York, NY, USA. 635-660.
- Luo Y, Teng F, Fu H, Ding GS. (2022). Immunotherapy in liver transplantation for hepatocellular carcinoma: Pros and cons. World J Gastrointest Oncol. 14:163-80.
[Crossref] [Google Scholar] [PubMed]
- Wei, X, Lijie X, Dilinigeer Z, Qiuyan C, Runqing L, Xiaoyu X, Jinyao L. (2019). The extracts of Artemisia absinthium L. suppress the growth of hepatocellular carcinoma cells through induction of apoptosis via endoplasmic reticulum stress and mitochondrial-dependent pathway. Molecules. 5:913.
[Crossref] [Google Scholar] [PubMed]
- Shaikh AM, Shrivastava DB, Apte KG, Navale SD. (2016). Medicinal plants as potential source of anticancer agents: A review. J Pharmacogn Phytochem. 5:291-95.
- JenÄa A, Mills DK, Ghasemi H, Saberian E, JenÄa A, Karimi Forood AM, Petrášová A, JenÄová J, Velisdeh ZJ, Zare-Zardini H. (2024). Herbal therapies for cancer treatment: A review of phytotherapeutic efficacy. Biologics. 18:229-55.
[Crossref] [Google Scholar] [PubMed]
- Abdel-Hamid NM, Abass SA, Mohamed AA, Hamid DM. (2018). Herbal management of hepatocellular carcinoma through cutting the pathways of the common risk factors. Biomed Pharmacother. 107:1246-1258.
[Crossref] [Google Scholar] [PubMed]
- Guo J, Yan W, Duan H, Wang D, Zhou Y, Feng D, Zheng Y, Zhou S, Liu G, Qin X. (2024). Therapeutic effects of natural products on liver cancer and their potential mechanisms. Nutrients. 16:1642.
[Crossref] [Google Scholar] [PubMed]
- Rawat D, Shrivastava S, Naik RA, Chhonker SK, Mehrotra A, Koiri RK. (2018). An overview of natural plant products in the treatment of hepatocellular carcinoma. Anticancer Agents Med Chem. 18:1838-1859.
[Crossref] [Google Scholar] [PubMed]
- Wang Y, Li J, Xia L. (2023). Plant-derived natural products and combination therapy in liver cancer. Front Oncol. 13:1116532.
[Crossref] [Google Scholar] [PubMed]
- Asma ST, Acaroz U, Imre K, Morar A, Shah SR, Hussain SZ, Arslan-Acaroz D, Demirbas H, Hajrulai-Musliu Z, Istanbullugil FR, Soleimanzadeh A. (2022). Natural products/bioactive compounds as a source of anticancer drugs. Cancers (Basel). 14:6203.
[Crossref] [Google Scholar] (All versions) [PubMed]
- Sharifi-Rad J, Herrera-Bravo J, Semwal P, Painuli S, Badoni H, Ezzat SM, Farid MM, Merghany RM, Aborehab NM, Salem MA, Sen S. (2022). Artemisia spp.: An update on its chemical composition, pharmacological and toxicological profiles. Oxid Med Cell Longev. 2022:5628601.
[Crossref] [Google Scholar] [PubMed]
- Wubuli A, Abdulla R, Zhao J, Wu T, Aisa HA. (2024). Exploring anti-inflammatory and antioxidant-related quality markers of Artemisia absinthium L. based on spectrum-effect relationship. Phytochem Anal. 35:1152-73.
[Crossref] [Google Scholar] [PubMed]
- El-Said KS, Haidyrah AS, Mobasher MA, Khayyat AI, Shakoori A, Al-Sowayan NS, Barnawi IO, Mariah RA. (2023). Artemisia annua extract attenuate doxorubicin-induced hepatic injury via PI-3K/Akt/Nrf-2-mediated signaling pathway in rats. Int J Mol Sci. 24:15525.
[Crossref] [Google Scholar] [PubMed]
- Li Y, Tian Y, Zhong W, Wang N, Wang Y, Zhang Y, Zhang Z, Li J, Ma F, Zhao Z, Peng Y. (2021). Artemisia argyi essential oil inhibits hepatocellular carcinoma metastasis viasuppression of DEPDC1 dependent Wnt/β-Catenin signaling pathway. Front Cell Dev Biol. 9:664791.
[Crossref] [Google Scholar] [PubMed]
- Kim J, Jung KH, Yan HH, Cheon MJ, Kang S, Jin X, Park S, Oh MS, Hong SS. (2018). Artemisia Capillaris leaves inhibit cell proliferation and induce apoptosis in hepatocellular carcinoma. BMC Complement Altern Med. 18:147.
[Crossref] [Google Scholar] [PubMed]
- Malhab LJB, Harb AA, Eldohaji L, Taneera J, AlâHroub HM, Abuhelwa A, Alzoubi KH, AbuâIrmaileh B, Hudaib M, Almaliti J, AbdelâRahman WM. (2024). Exploring the anticancer effect of Artemisia herba-alba on colorectal cancer: Insights from eight colorectal cancer cell lines. Food Sci Nutr. 13:e4715.
[Crossref] [Google Scholar] [PubMed]
- Tsamesidis I, Papadimitriou-Tsantarliotou A, Christodoulou A, Amanatidou D, Avgeros C, Stalika E, Bousnaki M, Michailidou G, Beketova A, Eleftheriou P, Bikiaris DN. (2024). Investigating the cytotoxic effects of Artemisia absinthium extract on oral carcinoma cell line. Biomedicines. 12:2674.
[Crossref] [Google Scholar] [PubMed]
- Jang E, Kim BJ, Lee KT, Inn KS, Lee JH. (2015). A survey of therapeutic effects of Artemisia capillaris in liver diseases. Evid Based Complement Alternat Med. 2015:728137.
[Crossref] [Google Scholar] (All versions) [PubMed]
- Blois MS. (1958). Antioxidant determinations by the use of a stable free radical. Nature. 181:1199-200.
- Hiai S, Oura H, Odaka Y, Nakajima T. (1975). A colorimetric estimation of ginseng saponins. Planta Medica. 28:363-69.
[Crossref] [Google Scholar] [PubMed]
- Singleton VL, Orthofer R, Lamuela-Raventós RM. (1999). Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. Methods Enzymol. 299:152-78.
- Prieto P, Pineda M, Aguilar M. (1999). Spectrophotometric quantitation of antioxidant capacity through the formation of a phosphor-molybdenum complex: Specific application to the determination of vitamin E. Anal. Biochem. 269:337-41.
[Crossref] [Google Scholar] (All versions) [PubMed]
- Weir NM, Selvendiran K, Kutala VK, Tong L, Vishwanath S, Rajaram M, Tridandapani S, Anant S. (2007). Curcumin induces G2/M arrest and apoptosis in cisplatin-resistant human ovarian cancer cells by modulating Akt and p38 MAPK. Cancer Biol Ther. 6:178-84.
[Crossref] [Google Scholar] [PubMed]
- Livak KJ, Schmittgen TD. (2001). Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCT method. Methods. 25:402-08.
[Crossref] [Google Scholar] [PubMed]
- Liu PH, Hsu CY, Hsia CY, Lee YH, Su CW, Huang YH, Lee FY, Lin HC, Huo TI. (2016). Prognosis of hepatocellular carcinoma: Assessment of eleven staging systems. J Hepatol. 64:601-08.
[Crossref] [Google Scholar] [PubMed]
- Sultan MH, Zuwaiel AA, Moni SS, Alshahrani S, Alqahtani SS, Madkhali O, Elmobark ME. (2020). Bioactive principles and potentiality of hot methanolic extract of the leaves from Artemisia absinthium L "in vitro cytotoxicity against human MCF-7 breast cancer cells, antibacterial study and wound healing activity". Curr Pharm Biotechnol. 21:1711-21.
[Crossref] [Google Scholar] [PubMed]
- Munteanu A, Gogulescu A, Èoica C, Mioc A, Mioc M, Milan A, Lukinich-Gruia AT, Pricop MA, Jianu C, Banciu C, Racoviceanu R. (2024). In vitro and in silico evaluation of Syzygium aromaticum essential oil: Effects on mitochondrial function and cytotoxic potential against cancer cells. Plants (Basel). 13:3443.
[Crossref] [Google Scholar] [PubMed]
- Renugadevi K, Julius A. (2020). GC-MS analysis of bioactive compounds of Artemesia annua and assessment of its anti-proliferative activity against human cancer cell lines. Int J Pharm Sci Res. 11:1840-43.
- Emami SA, Vahdati-Mashhadian N, Vosough R, Oghazian MB. (2009). The anticancer activity of five species of Artemisia on Hep2 and HepG2 cell lines. Pharmacology online. 3:327-39.
- Sharmila K, Padma PR. (2013). Anticancer activity of Artemisia vulgaris on hepatocellular carcinoma (HepG2) cells. Int J Pharm Pharm Sci. 5:479-83.
- Pace L, Ragusa F, Lizzi L, Armillotta MG, Massimi M. (2024). Potential antiproliferative and antimetastatic effects of Artemisia eriantha: An in vitro study focused on hepatocarcinoma cells. Biology (Basel). 13:985.
[Crossref] [Google Scholar] [PubMed]
- Williams GH, Stoeber K. (2012). The cell cycle and cancer. J Pathol. 226:352-64.
[Crossref] [Google Scholar] [PubMed]
- Koutb N, Ebeed N, Salem L, Girgis S, Ahmad E. (2021). The anticancer activity of Artemisia Judaica crude extract in human hepatocellular carcinoma HepG2 cells by induction of apoptosis and cell cycle arrest. IJCRR. 13:209-15.
- Kim HG, Lee SB, Lee JS, Kim WY, Choi SH, Son CG. (2017). Artemisia iwayomogi plus Curcuma longa synergistically ameliorates nonalcoholic steatohepatitis in HepG2 Cells. Evid Based Complement Alternat Med. 2017:4390636.
[Crossref] [Google Scholar] (All versions) [PubMed]
- Kim JG, Kim W, Kim KY. (2023). Alpinia japonica extract induces apoptosis of hepatocellular carcinoma cells through G0/G1 cell cycle arrest and activation of JNK. Cell Mol Biol (Noisy-le-grand). 69:12-18.
[Crossref] [Google Scholar] [PubMed]
- Sorriento D. (2024). Oxidative stress and inflammation in cancer. Antioxidants (Basel). 13:1403.
[Crossref] [Google Scholar] [PubMed]
- Ali A, Lim J, Kim EH, Lee JH, Seong S, Kim W. (2021). Antiâinflammatory effects of heatâprocessed Artemisia capillaris thunberg by regulating IκBα/NFâκB complex and 15âPGDH in mouse macrophage cells. Evid Based Complement Alternat Med. 2021:5320314.
[Crossref] [Google Scholar] [PubMed]
- Abate G, Zhang L, Pucci M, Morbini G, Mac Sweeney E, Maccarinelli G, Ribaudo G, Gianoncelli A, Uberti D, Memo M. (2021). Phytochemical analysis and anti-inflammatory activity of different ethanolic phyto-extracts of Artemisia annua L. Biomolecules. 11:975.
[Crossref] [Google Scholar] [PubMed]
- Lee J, Kim E, Jeong G. (2024). Anti-inflammatory herbal extracts and their drug discovery perspective in atopic dermatitis. Biomol Ther (Seoul). 32:25-37.
[Crossref] [Google Scholar] [PubMed]
- Èicolea M, Pop RM, Pârvu M, Usatiuc LO, UifÄlean A, Ranga F, Pârvu AE. (2024). Phytochemical composition antioxidant and anti-inflammatory activity of Artemisia dracunculus and Artemisia abrotanum. Antioxidants (Basel). 13:1016.
[Crossref] [Google Scholar] [PubMed]
- Èicolea M, Pop RM, Pârvu M, Usatiuc LO, UifÄlean A, Pop DD, Fischer-Fodor E, Ranga F, Rusu CC, CÄtoi AF, Palma-Garcia F. (2025). Flowers and leaves of Artemisia absinthium and Artemisia annua phytochemical characterization, anti-inflammatory, antioxidant, and anti-proliferative activities evaluation. Plants. 14:1029.
[Crossref] [Google Scholar] [PubMed]
- Cherfi I, Mahboub N, Toumi I, Laouini SE, Hasan GG, Bouafia A, Alharthi F, Emran TB. (2025). Assessment of Artemisia Campestris L. leaf extract effects on polycystic ovarian syndrome in rats, antioxidant and α-amylase inhibition activities. Chem. Biodivers. 22:e202402184.
[Crossref] [Google Scholar] [PubMed]
- Sun EJ, Wankell M, Palamuthusingam P, McFarlane C, Hebbard L. (2021). Targeting the PI3K/Akt/mTOR pathway in hepatocellular carcinoma. Biomedicines. 9:1639.
[Crossref] [Google Scholar] [PubMed]
- Chen G, Zhang Y, Zhou Y, Luo H, Guan H, An B. (2024). Targeting the mTOR pathway in hepatocellular carcinoma: The therapeutic potential of natural products. J Inflamm Res. 17:10421-10440.
[Crossref] [Google Scholar] [PubMed]
- Paskeh MD, Ghadyani F, Hashemi M, Abbaspour A, Zabolian A, Javanshir S, Razzazan M, Mirzaei S, Entezari M, Goharrizi MA, Salimimoghadam S. (2023). Biological impact and therapeutic perspective of targeting PI3K/Akt signaling in hepatocellular carcinoma: Promises and challenges. Pharmacol Res. 187:106553.
[Crossref] [Google Scholar] [PubMed]