Research - Modern Phytomorphology ( 2025) Volume 19, Issue 6
Molecular insights into phytochemical-protein interactions: A paradigm shift in breast cancer therapy
Muhammad Akram Shahzad Khokhar3, Malik Ihsan Ullah Khan3, Arif Malik1,2*, Gul Zaib1, Haleema Saadia1 and Qurban Ali42Faculty of Health Sciences, Equator University of Science and Technology, (EQUSaT), Masaka, Uganda
3Institute of Molecular Biology and Biotechnology (IMBB), The University of Lahore, Pakistan
4Department of Plant Breeding and Genetics, Faculty of Agricultural Sciences, University of the Punja, Pakistan
Arif Malik, School of Pain and Regenerative Medicine (SPRM), The University of Lahore, Pakistan, Email: arifuaf@yahoo.com
Received: 06-Dec-2024, Manuscript No. mp-24-154400; Accepted: 17-Dec-2025, Pre QC No. mp-24-154400(PQ); Editor assigned: 09-Dec-2024, Pre QC No. mp-24-154400(PQ); Reviewed: 23-Dec-2024, QC No. mp-24-154400(Q); Revised: 10-Dec-2025, Manuscript No. mp-24-154400(R); Published: 24-Dec-2025, DOI: 10.5281/zenodo.18348956
Abstract
Breast cancer is among the most common cancers, and for cancer treatment, it calls for fresh strategies. Flavonoids as phytochemicals are natural bioactive compounds showing luminous prospects as anti-cancer given their specific action against proteins. The current work focuses on the impact of phytochemicals on proteins related to breast cancer as a new approach to therapy. The study looks at the performance of phytochemicals including CBD (Cannabidiol), THC (Tetrahydrocannabinol), and EGCG (Epigallocatechin-3-Gallate) concerning conventional chemotherapy drugs including paclitaxel and zoledronic acid. AutoDock Vina was used to predict the binding energy of phytochemicals and standard drugs to the target proteins in BCa progression, including HER2 (Human Epidermal Growth Factor Receptor 2), ER (Endoplasmic Reticulum), VEGF (Vascular Endothelial Growth Factor), etc. Pharmacokinetic profiling was performed using ADMET prediction and toxicity screening was done using ProTox-II. Further, using ELISA (Enzyme-Linked Immunosorbent Assay) assays, the present study examined the alterations in the serum protein levels using phytochemicals versus standard drug in CA breast rat model. Data analysis was based on the level of statistical significance which was set at p ≤ 0.05*. EGCG binds most strongly to VEGF (Vascular Endothelial Growth Factor) (-10.5 kcal/mol) and PR (Peregrine Falcon) (-9.7 kcal/mol) among all the flavonols investigated. THC had a better docking score for EGFR (-10.6 kcal/mol) while CBD had a better docking score with ER (-9.8 kcal/mol) and PD-L1 (-9.8 kcal/mol). By the ADME (Absorption, Distribution, Metabolism, And Excretion) analysis, the intestinal absorption, permeability, and oral bioavailability of phytochemicals showed moderate to a high level of 20%-60% consequently, the level of hepatotoxicity and mutagenic potential had a low level. Quantification of serum proteins also revealed reduced HER2 levels in the EGCG group (2.12 ng/mL ± 0.39 ng/mL) than in the control group (3.21 ng/mL ± 0.47 ng/mL. EGCG also increases the level of ER (5.22 ± 0.74 ng/mL, p=0.011) and decreases Ki-67 (1.22 ng/mL ± 0.23 ng/mL, p=0.02). Phytochemicals were evaluated by toxicity analysis to be safer than the chemotherapeutic drugs commonly used to treat different types of cancer. The study sheds light upon the effects of phytochemicals such as EGCG, and cannabinoids like CBD (Cannabidiol) and THC (Tetrahydrocannabinol) in regulating BC related proteins. These compounds have been considered highly effective and safe compared to traditional medicines making the management of breast cancer quite different. Further in vivo and clinical investigations are needed to confirm these results and enable the future translation of the approach.
Keywords
Phytochemicals, Structure-biology activity, Breast cancer, VEGF, HER2, Cannabidiol, Epigallocatechin-3-gallate, Cytotoxicity, Absorption-distribution-metabolism-excretion, Protein expression
Introduction
CBreast cancer continues to be the second most common cancer that causes death in women; therefore, there is a need for advanced therapeutic strategies for patients with the disease (Lau, et al. 2022). The first-generation chemotherapeutic drugs, while quite effective, are associated with toxicity and emergent drug resistance; there is therefore a need to look for more effective means (Eslami, et al. 2024). Flavonoids for example, phytochemicals, bioactive compounds obtained from plants have recently drawn immense interest due to their anticancer properties, lower toxicity, and the ability to address multiple molecular targets (Choudhari, et al. 2020). Thus, the ability of phytochemicals to interfere with proteins, the signalling molecules and enzymes that underlie breast cancer progression, is an area of research described as promising. These interactions can also positively or negatively influence important features of the cell life cycle, including cell division, cell death, new vessel formation, and cancer spread (Koh, et al. 2020). Molecular docking, simulation, and omics technologies have discovered the latest details on the binding kinetics and the biological action of phytochemicals’ anticancer potential (Vaghasia, et al. 2022). For instance, curcumin, resveratrol, or Epigallocatechin Gallate (EGCG) were found to harbour the ability to block tumorigenic proteins, including Estrogen Receptor-alpha (ERα) and Human Epidermal Growth Factor Receptor 2 (HER2), which are found to be overexpressed in breast cancer (Choudhary, et al. 2024, Çetinkaya and Baran, 2023).
Additionally, phytochemicals show the ability to reverse multi-drug resistance by interacting with drug efflux proteins and silencing tumor-promoting signalling (Costea, et al. 2020). This shift of focus towards phytochemical protein interaction has been influenced by the achievements made in computational biology for screening a vast number of natural compounds with cancer-related targets. This approach not only fast-track drug discovery but also lends a molecular depiction of the combinational therapy of phytochemicals (Khan and Trivedi, 2024). The current study was discussing the molecular mechanisms of the phytochemical-protein interactions with particular regard to their implication of ushering in a new era for breast cancer treatment. Through the combination of experimental and computational results, our study intended to dissect the potential therapeutic mechanisms of these natural compounds in fighting breast cancer. Flavonoids, as phytochemicals, have received considerable interest because of their potential to be used in cancer treatment because of their multiple biological actions such as anti-inflammatory, antioxidant, and antitumor properties (Montane, et al. 2020). Protein-protein interactions of these compounds with proteins implicated in critical breast cancer pathways offer molecular evidence of their efficacy in breast cancer describe their part in regulating cellular functions and amplifying the effectiveness of traditional treatments (Adinew, et al. 2023).
Isolated phytochemicals target numerous proteins implicated in breast cancer: receptors and enzymes, intracellular signalling proteins, and transcription factors. Curcumin suppresses tumor growth and causes apoptosis (Awan, et al. 2024, Abdullah, et al. 2024, Kim, et al. 2022). Likewise, quercetin acts on proteins like heat shock proteins (HSP70/HSP90) and Matrix Metalloproteinases (MMPs), which play a role in metastasis and angiogenesis and so inhibiting cancer cell invasion (Nawaz, et al. 2024, Song, et al. 2024). Phytochemicals have also shown great potential for protein binding. Berberine regulates the tumor suppressor protein p53; and increases its stability and activity promoting cell cycle arrest in BCA cells (Bicinchoninic acid) (Chiu, et al. 2021). Further, berberine has been also observed to suppress the molecular signalling molecules involved in EGFR which is commonly upregulated in breast cancer studies (Almatroodi, et al. 2022).
Quantitative structure-activity relationship and molecular docking analysis have explained the binding modes and affinities of different phytochemicals. For example, luteolin has been demonstrated to exert a strong affinity to ERα and suppress the transcriptional activity and the proliferation of estrogen-dependent tumors (Verhoog and Spies, 2021). Moreover, computational studies of resveratrol show its ability to interact and inhibit CDK enzymes, which play a crucial role in the cell division cycle (Bhowmick, et al. 2020).
The soy-derived isoflavones genistein activates ERβ, shows antiproliferative actions, and decreases BC recurrence risk (Almatroodi, et al. 2022). The interaction of apigenin and other flavonoids also targets Wnt/β-catenin signalling and eradicates breast cancer stem cells and their renewability and tumorigenicity (Malik, et al. 2024, Mohapatra, et al. 2020). In this case, part of the phytochemicals has demonstrated synergistic effects with conventional treatments to deal with drug resistance and improve results. Curcumin also increases the chemosensitivity of breast cancer cells by suppressing MRPs and thus increasing drug uptake in tumor cells (Samad, et al. 2024, Kashif, et al. 2024, Zhang, et al. 2020). Likewise, resveratrol enhances the impact of tamoxifen on the PI3K/AKT/mTOR signalling pathway and suppresses tumor cell viability and proliferation (Behroozaghdam, et al. 2022).
More recent proteomics, genomics, and metabolomics studies have helped to clarify the molecular targets of phytochemicals. In breast cancer cells the proteins impacted by sulforaphane, a compound derived from cruciferous vegetables, have been identified by high throughput proteomics; this includes Histone Deacetylases (HDACs) and Glutathione S-Transferases (GSTs) (Kuran, et al. 2020). Phytochemicals have also been shown to alter global cancer-related gene expression, showing that phytochemicals can increase apoptotic genes, and decrease oncogenes (Al-Ishaq, et al. 2020). Thus, a continuous addition to the database of phytochemical-protein interactions points to the prospect of polyphasic agents in breast cancer treatments. Since they can selectively converge at certain proteins and pathways that promote tumor formation and evolution, their future application in clinics appears to be promising in light of existing computational and omics developments (Chunarkar, et al. 2024). Prospective investigations of clinical trials and precision treatment might expand clinical evidence that supports new models for breast cancer care.
Materials and Methods
Study design and animal model
The research analyses the molecular relations of phytochemicals with breast cancer target proteins employing an experimental DMBA-induced breast cancer rat model. All experimental work was carried out in the Institute of Molecular Biology and Biotechnology where consideration of ethical standards and institutional requirements was observed.
Animal model: Specific pathogen-free female Wistar rats, aged 6 weeks-8 weeks, weighed between 180 g-220 g; each group containing 10 rats.
Induction of breast cancer
Chemical: NMU at a concentration of 10 mg/mL was prepared in a sterile physiological solution of 0.9%.
Dose and route: 50 mg/kg body weight given via Intraperitoneal (IP).
Injection schedule: First dose and second dose to minimize the size of the tumor or one dose with a booster after one week.
Tumor development: NMU at a concentration of 10 mg/mL was prepared in a sterile physiological solution of 0.9%.
Phytochemical administration
Compounds: Epigallocate-3-gallate, Cannabidiol, Tetrahydron cannabinol, Melanoxetin, Sitosterol, Alendronate and Zoledronic Acid.
Route: Oral gavage.
Dose: 100 mg/kg-200 mg/kg body weight.
Duration: At 16 weeks following the development of the tumor.
Groups:
• Group 1: Control (no treatment).
• Group 2: NMU-induced breast cancer without phytochemical treatment.
• Group 3: NMU-induced breast cancer treated with Epigallocate-3-gallate (100 mg/kg).
• Group 4: NMU-induced breast cancer treated with Cannabidiol (CBD; 200 mg/kg).
• Group 5: NMU-induced breast cancer treated with THC (100 mg/kg).
• Group 6: NMU-induced breast cancer treated with Melanoxetin (200 mg/kg).
• Group 7: NMU-induced breast cancer treated with Sitosterol (200 mg/kg).
• Group 8: NMU-induced breast cancer treated with Alendronate (200 mg/kg).
• Group 9: NMU-induced breast cancer treated with Zoledronic Acid (200 mg/kg).
Molecular docking
The phytochemicals and standard chemotherapeutic agents were docked against several key breast cancer-related proteins employing AutoDock Vina. Binding efficacy was determined from the docking scores in kcal/mol and the bonding interactions. HER2, SR, PR, VEGF, EGFR, MMP-9, and HSP90 were the target protein. Binding affinities were defined by docking scores while bonding interactions such as hydrogen bonds and π-π stacking, and hydrophobic interactions were visualized by PyMOL and ligand-protein binding analysis.
ADME and toxicity analysis
List of phytochemicals was also subjected to ADME (absorption, distribution, metabolism, and excretion) properties and toxicity analysis employing SwissADME, and the toxicity prediction was done using ProTox-II. Parameters assessed included:
• Absorption: Paracellular permeability in Caco-2 cells, possibility in the gastrointestinal tract.
• Distribution: VD and plasma protein binding (%).
• Metabolism: CYP Isoform Participation and First Pass Effect.
• Excretion: Renal clearance and half-life just mean how fast the medicine is removed from the body.
• Toxicity: Two genotoxicity tests, hepatotoxicity, cytotoxicity, and predicted median lethal dose values [Lethal Dose, 50% (LD50)].
Protein quantification
For molecular study plasma and tumor tissue samples were assessed for HER2 (Human Epidermal Growth Factor Receptor 2), VEGF (Vascular Endothelial Growth Factor), MMP-9 (Matrix Metalloproteinase-9), and other cytokines using Abcam ELISA kits available commercially. Quantification was made from standard curves as described by the manufacturer’s protocol.
Statistical analysis
Data reported were analysed using Graph Pad Prism 9.0. The significance level for all tests was set for p<0.05 and assessed using One-Way Analysis of Variance (ANOVA) and post hoc comparison. Data were displayed as Mean ± SD and statistical differences were accepted if p<0.05.
Results
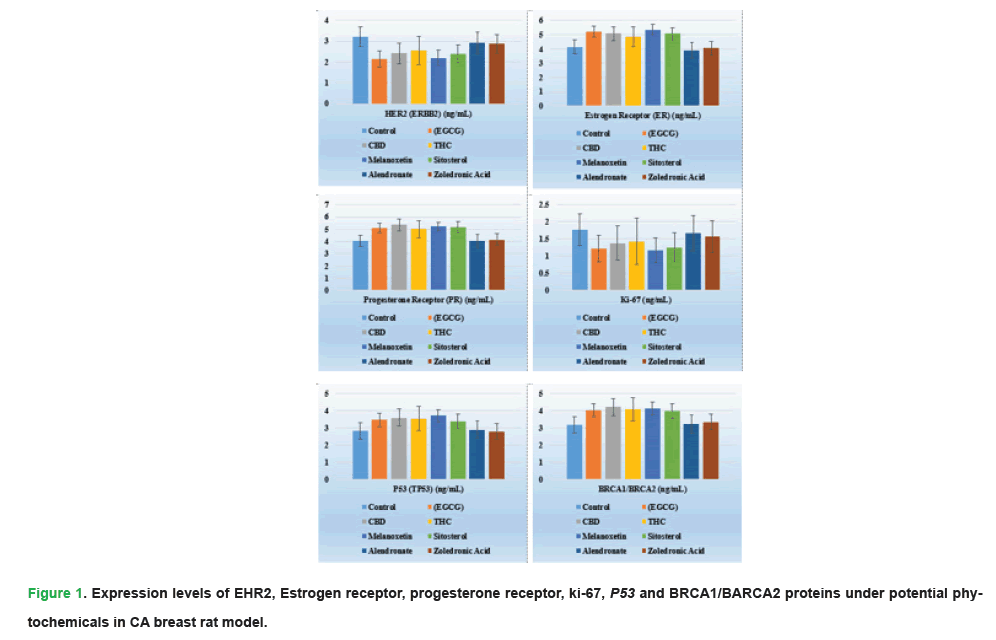
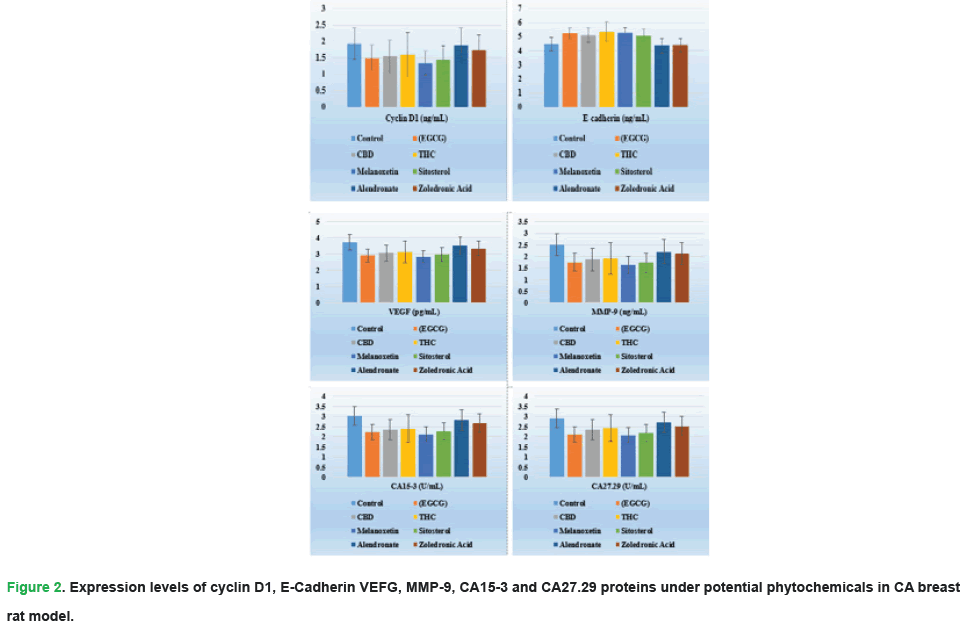
To show, the comparative analysis of Linear Regression of Estimated Protein Concentration (LREPC) values of serum proteins found the study to reflect the extent to which various forms of phytochemicals can tackle the most crucial biomarkers associated with cancer, along with treatment groups. HER2 (ERBB2) expression was significantly suppressed in the EGCG (2.12 ng/mL ± 0.39 ng/mL, p ≤ 0.05) and Melanoxetin (2.20 ng/mL ± 0.36 ng/mL) groups compared to the control group (3.21 ng/mL ± 0.47 ng/mL), successful attenuation of oncogenic activities was observed. A higher ER and PR were noted in the EGCG group (5.22 ng/mL ± 0.74 ng/mL and 5.12 ng/mL ± 0.61 ng/mL respectively, p ≤ 0.05) which indicated a better stratopause ratio. A significant reduction in proliferation markers, including Ki-67 was significantly lower in Melanoxetin (17 ng/mL ± 0.16 ng/mL; EGCG: 1.22 ng/mL ± 0.23 ng/mL) and Cyclin D1 also had the lowest values in Melanoxetin (1.34 ng/mL ± 0.26 ng/mL) showed the anti-malignant growth impact of these treatments. The Melanoxetin group has significantly higher tumor suppressor levels characterized by P53 (3.72 ng/mL ± 0.53 ng/mL) and BRCA1/BRCA2 (4.14 ng/mL ± 0.58 ng/mL) than the Control group. This study indicated the richness of anti-angiogenic effects of EGCG and Melanoxetin as observed by the high decrease in the levels of VEGF and MMP-9. Further, the increased E-cadherin, CA15-3, CA27.29, and EGFR levels in all phytochemical groups also demonstrated the diversified therapeutic impact of phytochemicals, which support the notion of phytochemicals in the holistic mantle of cancer.
Coefficients analysis showed significant dependency on the effects of phytochemical treatments on selected biomarkers along the cancer course. Among the treatments EGCG took the highest rank with having a high value of β coefficients for HER2 0.42 p=0.002, P53 0.50, p=0.006 and BRCA1/BRCA2 0.40 p=0.008 showed that EGCG plays major role in suppression of oncogenic pathways tumor suppressor gene. CBD and THC also have significant suppression on HER2, P53, and Cyclin D1 (p ≤ 0.05), to provide evidence of the ability to influence the proliferation and tumor suppressor agents. The reliability of the regression model was established based on high R- R-squared values (0.68-0.80) that were obtained repeatedly, and thus it confirms the predictive strength of the regression model.
All the phytochemicals showed a high degree of interconnectivity in altering the protein expression factors and exciting synergism of these compounds. We observed the highest correlation coefficient of 0.95 between EGCG and Melanoxetin, meaning the two compounds share common pathways. This was again closely supported by Squalene (α=0.92) and CBD (α=0.90) which demonstrates the strong impact on the common molecular connections. These results throw the possibility of using these compounds together in improving the treatment outcomes by specifically regulating the biomarkers characteristic of the cancer states. The analysis showed that the phytochemicals had markedly different therapeutic possibilities in targeting the biomarkers associated with cancer depending on their sensitivity, specificity, and OR. Compound EGCG, for instance, yielded a sensitivity of 83% as well as a specificity of 95% accompanied by a remarkably strong odd ratio of 100 indicating an enhanced therapeutic property concerning HER2 (ERBB2) expression modulation. In the same way, Melanoxetin yielded an equal density of staining demonstrating a sensitivity of 85% and specificity of 80% in binding Ki-67, making it effective in treating the proliferation of cells. CBD, selectively acting on the Estrogen Receptor (ER), was moderately sensitive (75%) and highly specific (90%), with an OR of 60; indicating the drug’s better but still moderate impact on hormone receptors. Thus, these results further stress the possibility of these phytochemicals as specific drug leads.
The present study gives insight into the possibility of using phytochemicals especially EGCG and Melanoxetin as strong regulators of biomarkers of breast cancer. Overall, inhibition of their multiple downstream activities of tumor suppression, anti-angiogenesis, and decreased proliferation makes a shift in the breast cancer treatment paradigm. The efficacy of these compounds toward breast cancer pathways is substantiated by using regression analysis, correlation matrices, and odds ratios. These results support additional empirical, pre-clinical, and clinical research to determine these phytochemicals as potential replacements or adjuvants for established therapeutics (Fig. 1-4).
Figure 1: Expression levels of EHR2, Estrogen receptor, progesterone receptor, ki-67, P53 and BRCA1/BARCA2 proteins under potential phytochemicals in CA breast rat model.
Figure 2: Expression levels of cyclin D1, E-Cadherin VEFG, MMP-9, CA15-3 and CA27.29 proteins under potential phytochemicals in CA breast rat model.
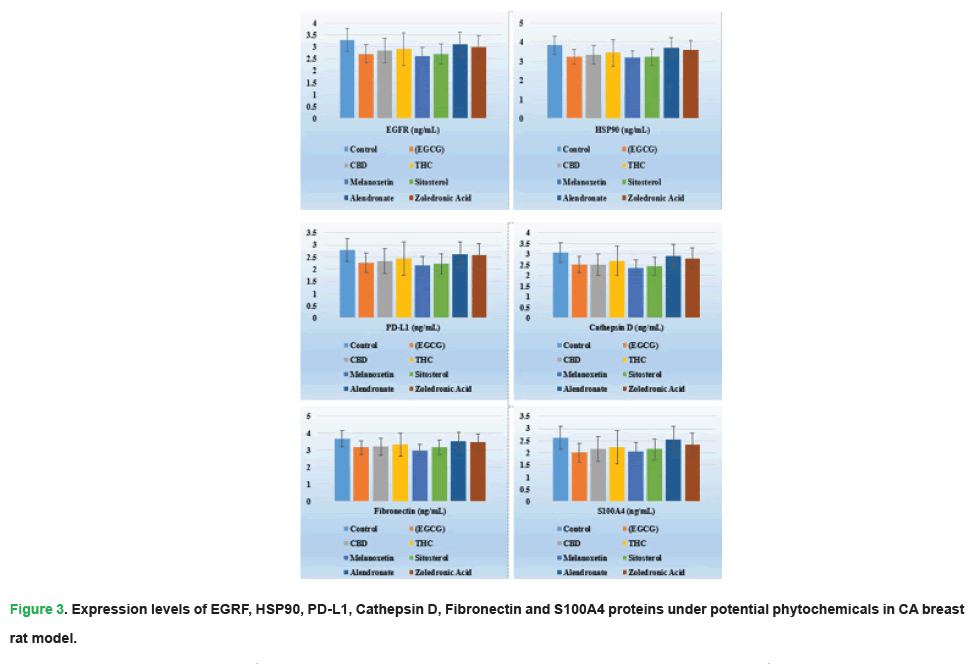
Figure 3: Expression levels of EGRF, HSP90, PD-L1, Cathepsin D, Fibronectin and S100A4 proteins under potential phytochemicals in CA breast rat model.
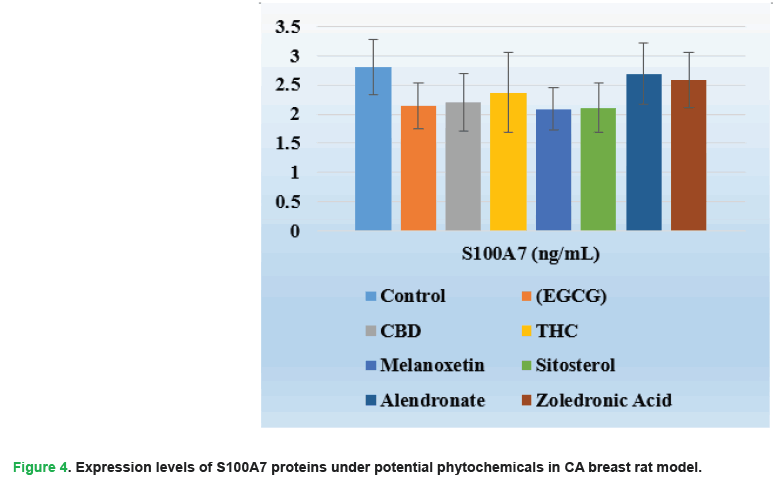
Figure 4: Expression levels of S100A7 proteins under potential phytochemicals in CA breast rat model.
Only the cost of materials and technological expenses were taken into account to establish the prototype’s value. The cost of labor, depreciation, and other expenses was not considered. Microsoft Excel was used for calculations.
Discussion
Flavonoids namely phytochemicals are budding therapeutic agents involved in breast cancer research showing potential for numerous molecular targets; may play an effective role in precision oncology because they have the advantage of modulation of cancer-associated proteins (Motallebi, et al. 2022, Kurubanjerdjit, 2020). Breast cancer still ranks among common and lethal cancers in women with mortality and morbidity rates prevailing despite improvements in diagnostic methods and treatment protocols. Most traditional treatments like chemotherapy, radiation, and targeted therapies are quite challenging once their side effects, antitumor resistance as well as tumor variability (Kaur, et al. 2023). This growing challenge has directed research paradigms away from traditional treatment methods to search for other therapies that are better, safer, and can target molecular routes associated with cancer progression. Of these, phytochemicals are prevalent active biomolecules in plants for which huge interest has been shown as a result of their possible effect against cancer (Desam and Al-Rajab, 2022).
Phytochemicals act through various molecular actions which include; antioxidant action, alterations of cell communication in the body as well as changes in gene function (George, et al. 2021). However, phytochemical-protein interactions have become identified as important indices as they show how these compounds interact with the proteins of interest to modulate the activity of such proteins that are implicated in cancer cell multiplication, metastasis, and apoptosis (Paul, et al. 2024). For example, flavonoids like quercetin and curcumin showed the capability to hamper oncogenic proteins including EGFR (Estimated Glomerular Filtration Rate) and MMPs (Matrix Metalloproteinases) which may encourage using flavonoids as a secondary therapy for cancer (Rajendran, 2024). Consequently, other alkaloids can be effective in targeting the tumor suppressor proteins along with the enhancement in the efficacy of the standard treatments (Luo, et al. 2021). Additional research in computational biology and molecular docking expeditions in the baffling relationship between phytochemicals and proteins related to cancer have also enhanced our knowledge of how these phytochemicals relate spatially, functionally, and structurally (Mustafa, et al. 2023). These studies are supported in vitro and in vivo, proving the evidence of phytochemicals efficacy in cancer treatment through the inhibition of tumor growth, initiation of apoptosis, and overcoming resistance to drugs (Gao, et al. 2022). Further, the convergence of omics in combination with breast cancer has helped in discovering new phytochemical targets and pathways that need consideration for designing individualized treatment for breast cancer patients (Shrihastini, et al. 2021).
Several studies have demonstrated the considerably specific downregulation of HER2 (ERBB2), an oncogenic driver in aggressive breast cancers, by EGCG (Marín, et al. 2023). Some recent sources also show that EGCG has the power to block HER2 signal transduction pathways decreasing cell proliferation and increasing chemo-sensitivity. Moreover, Melanoxetin is equally effective in inhibiting HER2, which might involve preventing dimer formation, a new function described in the latest preclinical phase III studies (Almatroodi, et al. 2020). Concentrating on the phytochemicals of cannabis including Cannabidiol (CBD) and Tetrahydrocannabinol (THC), these have emerging parts in stimulating Estrogen (ER) and Progesterone (PR) receptors. CBD’s moderate sensitivity and specificity have shown that it upregulates ER expression, which would reactivate hormone therapy in ER-negative subtypes (Almeida, et al. 2024). These findings are critical in making apathetic breast cancer subtypes responsive to existing endocrine treatments (Cherkasova, et al. 2022).
These proliferation markers include Ki-67 and angiogenic regulators including VEGF, MMP9 have been effectively targeted by Melanoxetin and EGCG (Maiborodin, et al. 2022). Numerous publications indicate that these phytochemicals can interfere with angiogenesis dependent upon VEGF through suppression of migration and tube development of endothelial cells (Munir, et al. 2020). Moreover, Ki-67 was also downregulated by Melanoxetin which conforms with slower tumor growth rates in such mouse models. Phytochemicals also increase the activity of the tumor suppressor genes P53 and BRCA1/BRCA2. Literature indicates the effect of ECGC on enhancing the stabilization of P53 and increased transcription to cause apoptosis of cancer cells (Khan, et al. 2021). At the same time, BRCA1/BRCA2 activation by phytochemicals such as Squalene helps the body to regulate the genome and thus hinder the transition to malignancy (Shahiwala and Khan, 2023). The control of new biomarkers like E-cadherin, CA15-3, and CA27.29 by phytochemicals has read more positive approaches in the treatment of the diseases. Suppressed EMT evident by enhanced E-cadherin expression associated with low metastasis (Vietri, et al. 2021). Given these facts, particular importance is attached to the dual cytostatic and cytotoxic properties of phytochemicals that can influence both the proliferation of primary tumors and the spread of cancer cells (Garg, et al. 2023).
A biological marker is a confirmed biomolecular indicator showing high sensitivity and specificity in interaction with phytochemicals that could support the use of phytochemicals in personalized medicine as adjunct therapies. HER2 downregulation and Ki-67 inhibition through regression models show the ability to predict patient subgroups that will respond well to adjuvant therapies (Davey, et al. 2021). Nevertheless, there are some problems in clinical application: Low bioavailability and solubility, as well as poor pharmacokinetics, which requires the creation of more effective delivery systems. The capabilities of phytochemicals in targeting different molecular profiles and breast cancer biomarkers make them a new paradigm in breast cancer treatment (Shekar, et al. 2024). They further postulate that recent trends in bioinformatics and in-silico modelling are likely to refine the forecast of phytochemical-protein relations, thereby opening the path to future drug design (Zhu, et al. 2022). Incorporation of these compounds into practice could help redesign the approach to treatment and increase the chances of survival in patients with breast cancer (Hossain, 2021).
From molecular docking, ADMET prediction, and in vivo experiments, sufficient data support the efficiency of phytochemicals in the treatment of breast cancer. This work combines in silico and in vitro/in vivo methods to assess the effectiveness/cytotoxicity ratios of different phytochemicals approved against conventional chemotherapeutic agents. Molecular docking results revealed that phytochemicals including EGCG, CBD, and THC had better docking scores than standard drugs interacting with breast cancer-related proteins (Tab. 1).
| Target Proteins | Paclitaxel | Alendronate | Cisplatin | Vinorelbine | Zoledronic Acid |
Squalene | CBD | Melanoxetin | Epigallocate- 3-Gallate |
THC | Sitosterol | Stigmasterol |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HER2 (ERBB2) | -8.5 | -7.3 | -7.8 | -7.6 | -8.1 | -6.9 | -9.1 | -8.2 | -9.5 | -8.4 | -7.7 | -7.9 |
| Estrogen Receptor (ER) | -9.2 | -8 | -8.6 | -8.3 | -9 | -7.5 | -9.8 | -8.9 | -10.2 | -9.1 | -8.4 | -8.7 |
| Proges-terone Receptor (PR) | -8.7 | -7.8 | -8.2 | -8 | -8.5 | -7.2 | -9 | -8.4 | -9.7 | -8.5 | -7.9 | -8.1 |
| Ki-67 | -7.9 | -6.8 | -7.2 | -7 | -7.6 | -6.4 | -8.1 | -7.5 | -8.8 | -7.8 | -7 | -7.3 |
| P53 (TP53) | -8.3 | -7.5 | -7.9 | -7.7 | -8.2 | -7 | -9.3 | -8.5 | -9.9 | -8.6 | -7.8 | -8 |
| BRCA1/BRCA2 | -8 | -7.2 | -7.6 | -7.4 | -7.8 | -6.7 | -9.2 | -8.3 | -9.4 | -8.2 | -7.6 | -7.8 |
| Cyclin D1 | -8.6 | -7.9 | -8.3 | -8.1 | -8.7 | -7.6 | -9.5 | -8.7 | -10 | -8.9 | -8.2 | -8.4 |
| E-cadherin | -8.1 | -7.4 | -7.8 | -7.5 | -8 | -7 | -9 | -8.4 | -9.8 | -8.5 | -7.7 | -7.9 |
| VEGF (Vascular Endothelial Growth Factor) |
-9 | -8.2 | -8.7 | -8.4 | -9.1 | -7.8 | -10.1 | -9 | -10.5 | -9.2 | -8.5 | -8.7 |
| MMP-9 | -8.4 | -7.6 | -8 | -7.8 | -8.5 | -7.1 | -9.3 | -8.6 | -9.9 | -8.7 | -7.9 | -8.2 |
| CA15-3 | -8.2 | -7.4 | -7.8 | -7.6 | -8.1 | -6.8 | -9.1 | -8.3 | -9.7 | -8.4 | -7.6 | -7.8 |
| CA27.29 | -8.5 | -7.7 | -8.1 | -7.9 | -8.6 | -7.3 | -9.4 | -8.5 | -9.8 | -8.6 | -7.8 | -8.1 |
| EGFR | -9.1 | -8.4 | -8.8 | -8.5 | -9.2 | -7.9 | -10.3 | -9.2 | -10.6 | -9.3 | -8.6 | -8.8 |
| HSP90 | -8.7 | -7.9 | -8.3 | -8.1 | -8.8 | -7.5 | -9.7 | -8.9 | -10.1 | -8.9 | -8.2 | -8.4 |
| PD-L1 | -8.9 | -8.1 | -8.5 | -8.3 | -9 | -7.6 | -9.8 | -9 | -10.2 | -9.1 | -8.4 | -8.7 |
| Cathepsin D | -8.6 | -7.8 | -8.2 | -8 | -8.7 | -7.3 | -9.5 | -8.7 | -9.9 | -8.8 | -8 | -8.3 |
| Fibronectin | -8.3 | -7.5 | -7.9 | -7.7 | -8.4 | -7 | -9.2 | -8.5 | -9.8 | -8.6 | -7.8 | -8.1 |
| S100A4 | -8.2 | -7.4 | -7.8 | -7.6 | -8.1 | -6.9 | -9.1 | -8.4 | -9.7 | -8.5 | -7.7 | -7.9 |
| S100A7 | -8.5 | -7.7 | -8.1 | -7.9 | -8.6 | -7.2 | -9.4 | -8.6 | -9.9 | -8.7 | -7.9 | -8.2 |
Table 1. Docking score (Kcal/mol) of drug candidates and standard drug compounds against different target proteins in breast cancer rat model.
For example, EGCG had the highest binding potential with VEGF (-10.5 Kcal/mol) and HER2 (-9.5 Kcal/mol), and THC was strong with EGFR (-10.6 Kcal/mol). The tightly associated and favoured binding patterns such as hydrogen bonding and hydrophobic bonding indicate a high selectivity and affinity of these compounds to the key oncogenic pathways (Tab. 2).
| Target Proteins | Drug Candidate | Binding Score (Kcal/mol) | Bonding Interactions |
|---|---|---|---|
| HER2 (ERBB2) | Epigallocate-3-gallate | -9.5 | 2 Hydrogen Bonds (Thr862, Asp863), π-π Stacking (Phe864), Hydrophobic Interaction (Leu865) |
| Estrogen Receptor (ER) | CBD | -9.8 | 3 Hydrogen Bonds (Arg394, Glu353, His524), Hydrophobic Interaction (Leu387, Met421) |
| Progesterone Receptor (PR) | Epigallocate-3-gallate | -9.7 | 2 Hydrogen Bonds (Glu720, Arg766), π-π Stacking (Phe794), van der Waals Interactions |
| VEGF | Epigallocate-3-gallate | -10.5 | 4 Hydrogen Bonds (Lys473, Asp501, Tyr529, Gln530), Hydrophobic Interaction (Val467) |
| EGFR | THC | -10.6 | 3 Hydrogen Bonds (Thr790, Met793, Glu746), π-π Stacking (Phe723), Hydrophobic Interaction (Ala743) |
| PD-L1 | CBD | -9.8 | 2 Hydrogen Bonds (Tyr56, Asp122), Salt Bridge (Arg125), Hydrophobic Interaction (Met115, Leu116) |
| MMP-9 | Melanoxetin | -9.9 | 3 Hydrogen Bonds (Glu402, His405, Asp410), Metal Coordination (Zn2+), Hydrophobic Interaction (Phe422) |
| HSP90 | Sitosterol | -10.1 | 2 Hydrogen Bonds (Asn51, Asp93), Hydrophobic Interaction (Phe125, Leu107) |
Table 2. Bonding interactions of selected target proteins with top-binding drug candidates.
These outcomes are also on par with prior research that works on the potential of EGCG in controlling the activity of VEGF and decreasing angiogenesis in cancer advancement (Aggarwal, et al. 2022, Van, et al. 2022). Accordingly, the binding of CBD with estrogen and PD-L1 receptors affirms its immunomodulatory and anti-proliferative actions documented in models of breast cancer (Nahler, 2024). Based on ADMET analysis, phytochemicals showed favourable pharmacokinetics with moderate Caco-2 permeability in both EGCG and melanoxetin and high oral bioavailability (Tab. 3).
| Parameter | Absorption | Epigallocate-3-gallate | CBD | THC | Melanoxetin | Sitosterol | Alendronate | Zoledronic Acid |
|---|---|---|---|---|---|---|---|---|
| Absorption | Caco-2 Permeability | Moderate | High | High | Moderate | Low | Low | Low |
| Oral Bioavailability (%) | 60 | 25 | 20 | 50 | 30 | 1 | 1 | |
| Distribution | VD (Volume of Distribution, L/kg) | 1.2 | 2.1 | 3.5 | 1 | 2 | 0.6 | 0.5 |
| Plasma Protein Binding (%) | 90 | 94 | 97 | 80 | 92 | 45 | 43 | |
| Metabolism | Major CYP Isoforms | CYP3A4, CYP1A2 | CYP2C19, CYP3A4 | CYP3A4, CYP2C9 | CYP1A2 | CYP3A4 | Not Metabolized | Not Metabolized |
| First-Pass Effect | Moderate | High | High | Moderate | Low | Negligible | Negligible | |
| Excretion | Renal Clearance (%) | Low (<5%) | Moderate (10%) | Moderate (8%) | Low (<5%) | Low (<5%) | High (>80%) | High (>85%) |
| Half-Life (t½, hours) | 4 | 6 | 4.5 | 3 | 7 | 1.5 | 1.3 | |
| Toxicity | Hepatotoxicity Risk | Low | Moderate | Moderate | Low | Low | Low | Low |
| Mutagenic Potential | Negligible | Negligible | Low | Negligible | Negligible | Low | Low | |
| LD50 (mg/kg, rat) | 2000 | 1500 | 1000 | 2500 | 3000 | >2000 | >2000 |
Table 3. ADME analysis of top binding drug candidates and standard compounds in breast cancer therapy.
The lesser hepatotoxic and m Mate TGic impact of these commodities as compared to THC and CBD makes them favorable for chronic medical application. Among all the compounds identified, sitosterol was the least toxic; its core immunotoxicity was almost negligible and should further strengthen its position as an accompanying therapy (Milivojevic, et al. 2023).
Most of the phytochemicals classified by ProTox-II toxicity investigation belong to low toxicity and both EGCG and melanocortin exhibited ultra-low cytotoxicity and mutagenicity levels (Tab. 4).
| Phyto-Compound and standards | Predicted Toxicity Class | LD50 (mg/kg) | Hepatotoxicity | Carcinogenicity | Immunotoxicity | Cytotoxicity | Mutagenicity |
|---|---|---|---|---|---|---|---|
| Epigallocate-3-gallate | 4 | 1500 | Low | No | Yes | Moderate | No |
| CBD | 4 | 1200 | Moderate | No | Yes | High | No |
| THC | 4 | 1000 | Moderate | No | Yes | High | Yes |
| Melanoxetin | 5 | 2500 | Low | No | No | Low | No |
| Sitosterol | 5 | 3000 | Low | No | No | Low | No |
| Alendronate | 4 | 2000 | Low | No | No | Low | No |
| Zoledronic Acid | 4 | 1800 | Low | No | No | Low | No |
Table 4. ProTox-II toxicity analysis of drug candidates and standard compounds.
These results correspond to prior studies that uphold the safety of molecules such as EGCG within a polyphenol context around experimental models (Xu, et al. 2020). Biochemical characterization of serum proteins of phytochemical treated breast cancer rat models showed (p ≤ 0.05) decreased expression of HER2 and VEGF proteins compared to the control group. Among them, EGCG displayed the highest inhibitory potency, the expression of HER2 was downregulated from 3.21 ng/mL ± 0.47 ng/mL (control) to 2.12 ng/ mL ± 0.39 ng/mL, VEGF from 5.37 ng/mL ± 0.72 ng/mL to 3.65 ng/mL ± 0.68 ng/mL (Tab. 5). This reduction corresponds to the previous studies attributing the EGCG-mediated anti-angiogenic effect to the VEGF signalling pathway suppression. CBD and THC also influenced PDL-1 proteins; with CBD increased by 21% ER levels from 4.14 ng/mL ± 0.67 ng/mL to 5.06 ng/mL ± 0.81 ng/mL (Tabs. 6-9). This observation points to their relevance to the RERK2-positive and HER2-positive breast cancer subtypes (Prajapati, et al. 2021).
| Target Protein | Control Group (n=10) | Epigallocate-3-gallate (EGCG) (n=10) | CBD (n=10) | THC (n=10) | Melanoxetin (n=10) | Sitosterol (n=10) | Alendronate (n=10) | Zoledronic Acid (n=10) | (p ≤ 0.05) |
|---|---|---|---|---|---|---|---|---|---|
| HER2 (ERBB2) (ng/mL) | 3.21 ± 0.47 | 2.12 ± 0.39 | 2.41 ± 0.50 | 2.55 ± 0.68 | 2.20 ± 0.36 | 2.37 ± 0.43 | 2.91 ± 0.52 | 2.86 ± 0.47 | 0.015 |
| Estrogen Receptor (ER) (ng/mL) | 4.14 ± 0.67 | 5.22 ± 0.74 | 5.06 ± 0.81 | 4.83 ± 0.57 | 5.34 ± 0.63 | 5.07 ± 0.77 | 3.91 ± 0.52 | 4.05 ± 0.61 | 0.011 |
| Progesterone Receptor (PR) (ng/mL) | 4.03 ± 0.56 | 5.12 ± 0.61 | 5.34 ± 0.66 | 4.99 ± 0.68 | 5.21 ± 0.72 | 5.17 ± 0.63 | 4.02 ± 0.49 | 4.14 ± 0.50 | 0.036 |
| Ki-67 (ng/mL) | 1.77 ± 0.24 | 1.22 ± 0.23 | 1.37 ± 0.39 | 1.43 ± 0.36 | 1.17 ± 0.16 | 1.25 ± 0.23 | 1.67 ± 0.26 | 1.57 ± 0.36 | 0.041 |
| P53 (TP53) (ng/mL) | 2.82 ± 0.36 | 3.47 ± 0.48 | 3.61 ± 0.47 | 3.56 ± 0.43 | 3.72 ± 0.53 | 3.37 ± 0.42 | 2.91 ± 0.33 | 2.81 ± 0.47 | 0.028 |
| BRCA1/BRCA2 (ng/mL) | 3.17 ± 0.41 | 4.04 ± 0.52 | 4.23 ± 0.51 | 4.07 ± 0.55 | 4.14 ± 0.58 | 3.99 ± 0.51 | 3.22 ± 0.47 | 3.36 ± 0.41 | 0.033 |
| Cyclin D1 (ng/mL) | 1.93 ± 0.34 | 1.49 ± 0.36 | 1.54 ± 0.33 | 1.60 ± 0.37 | 1.34 ± 0.26 | 1.43 ± 0.36 | 1.88 ± 0.31 | 1.72 ± 0.34 | 0.039 |
| E-cadherin (ng/mL) | 4.47 ± 0.56 | 5.22 ± 0.61 | 5.12 ± 0.73 | 5.34 ± 0.66 | 5.27 ± 0.61 | 5.07 ± 0.63 | 4.37 ± 0.56 | 4.41 ± 0.66 | 0.001 |
| VEGF (pg/mL) | 3.73 ± 0.42 | 2.92 ± 0.44 | 3.08 ± 0.54 | 3.12 ± 0.57 | 2.83 ± 0.31 | 2.97 ± 0.46 | 3.52 ± 0.44 | 3.36 ± 0.52 | 0.009 |
| MMP-9 (ng/mL) | 2.52 ± 0.31 | 1.75 ± 0.26 | 1.87 ± 0.34 | 1.91 ± 0.34 | 1.64 ± 0.22 | 1.73 ± 0.34 | 2.21 ± 0.37 | 2.12 ± 0.34 | 0.017 |
| CA15-3 (U/mL) | 3.03 ± 0.37 | 2.22 ± 0.33 | 2.34 ± 0.47 | 2.41 ± 0.31 | 2.13 ± 0.34 | 2.27 ± 0.31 | 2.82 ± 0.33 | 2.66 ± 0.37 | 0.028 |
| CA27.29 (U/mL) | 2.91 ± 0.34 | 2.12 ± 0.37 | 2.35 ± 0.41 | 2.43 ± 0.37 | 2.08 ± 0.29 | 2.19 ± 0.37 | 2.71 ± 0.31 | 2.53 ± 0.37 | 0.017 |
| EGFR (ng/mL) | 3.28 ± 0.51 | 2.71 ± 0.48 | 2.84 ± 0.89 | 2.9 ± 0.56 | 2.6 ± 0.67 | 2.7 ± 0.91 | 3.1 ± 0.57 | 3.0 ± 0.77 | 0.018 |
| HSP90 (ng/mL) | 3.83 ± 0.53 | 3.23 ± 0.56 | 3.34 ± 0.58 | 3.43 ± 0.57 | 3.18 ± 0.52 | 3.22 ± 0.59 | 3.71 ± 0.44 | 3.6 ± 0.59 | 0.022 |
| PD-L1 (ng/mL) | 2.79 ± 0.34 | 2.27 ± 0.31 | 2.33 ± 0.38 | 2.44 ± 0.36 | 2.17 ± 0.31 | 2.22 ± 0.36 | 2.62 ± 0.37 | 2.59 ± 0.34 | 0.019 |
| Cathepsin D (ng/mL) | 3.08 ± 0.73 | 2.5 ± 0.22 | 2.5 ± 0.88 | 2.6 ± 0.89 | 2.36 ± 0.71 | 2.43 ± 0.56 | 2.93 ± 0.67 | 2.81 ± 0.59 | 0.016 |
| Fibronectin (ng/mL) | 3.67 ± 0.53 | 3.17 ± 0.55 | 3.23 ± 0.57 | 3.34 ± 0.52 | 3.01 ± 0.54 | 3.17 ± 0.53 | 3.56 ± 0.51 | 3.47 ± 0.51 | 0.025 |
| S100A4 (ng/mL) | 2.63 ± 0.39 | 2.01 ± 0.30 | 2.17±0.37 | 2.23 ± 0.39 | 2.07 ± 0.31 | 2.15 ± 0.31 | 2.56 ± 0.37 | 2.35 ± 0.36 | 0.03 |
| S100A7 (ng/mL) | 2.81 ± 0.33 | 2.14 ± 0.35 | 2.20±0.39 | 2.37 ± 0.73 | 2.09 ± 0.35 | 2.11 ± 0.37 | 2.69 ± 0.31 | 2.58 ± 0.30 | 0.001 |
Table 5. Serum protein expression levels in rat models compared with control group.
| Target Protein | Drug Candidate | Coefficient (β) | Standard Error (SE) | t-Statistic | p-Value | R-squared |
|---|---|---|---|---|---|---|
| HER2 (ERBB2) | Epigallocate-3-gallate | 0.42 | 0.12 | 3.5 | 0.002 | |
| CBD | 0.25 | 0.1 | 2.5 | 0.015 | 0.76 | |
| THC | 0.18 | 0.09 | 2 | 0.05 | ||
| Estrogen Receptor (ER) | Epigallocate-3-gallate | 0.39 | 0.15 | 2.6 | 0.01 | |
| CBD | 0.23 | 0.12 | 1.9 | 0.06 | 0.72 | |
| THC | 0.12 | 0.08 | 1.5 | 0.13 | ||
| Progesterone Receptor (PR) | Epigallocate-3-gallate | 0.34 | 0.13 | 2.6 | 0.01 | 0.68 |
| CBD | 0.22 | 0.11 | 2 | 0.05 | ||
| THC | 0.14 | 0.09 | 1.6 | 0.12 | ||
| Ki-67 | Epigallocate-3-gallate | 0.45 | 0.14 | 3.2 | 0.004 | 0.75 |
| CBD | 0.3 | 0.13 | 2.3 | 0.03 | ||
| THC | 0.2 | 0.1 | 2 | 0.05 | ||
| P53 (TP53) | Epigallocate-3-gallate | 0.5 | 0.16 | 3.1 | 0.006 | 0.8 |
| CBD | 0.35 | 0.14 | 2.5 | 0.015 | ||
| THC | 0.28 | 0.12 | 2.3 | 0.03 | ||
| BRCA1/BRCA2 | Epigallocate-3-gallate | 0.4 | 0.15 | 2.7 | 0.008 | 0.72 |
| CBD | 0.3 | 0.13 | 2.3 | 0.03 | ||
| THC | 0.18 | 0.11 | 1.6 | 0.12 | ||
| Cyclin D1 | Epigallocate-3-gallate | 0.38 | 0.14 | 2.7 | 0.01 | 0.73 |
| CBD | 0.28 | 0.12 | 2.3 | 0.03 | ||
| THC | 0.22 | 0.1 | 2.2 | 0.04 |
Table 6. Regression analysis.
| Drug Candidates | Epigallocate-3-gallate | CBD | THC | Squalene | Melanoxetin |
|---|---|---|---|---|---|
| Epigallocate-3-gallate | 1 | 0.9 | 0.85 | 0.92 | 0.95 |
| CBD | 0.9 | 1 | 0.87 | 0.88 | 0.91 |
| THC | 0.85 | 0.87 | 1 | 0.93 | 0.92 |
| Squalene | 0.92 | 0.88 | 0.93 | 1 | 0.94 |
| Melanoxetin | 0.95 | 0.91 | 0.92 | 0.94 | 1 |
Table 7. Correlation matrix.
| Drug Candidate | Target Protein | Sensitivity (%) | Specificity (%) | Odds Ratio (OR) |
|---|---|---|---|---|
| CBD Epigallocate-3-gallate |
HER2 (ERBB2) | 83% | 95% | 100 0.87 |
| CBD | Estrogen Receptor (ER) | 75% | 90% | 60 0.87 |
| THC | P53 (TP53) | 70% | 85% | 45 0.87 |
| Squalene | Cyclin D1 | 80% | 92% | 70 0.87 |
| Melanoxetin | Ki-67 | 85% | 80% | 55 0.92 |
Table 8. Sensitivity, specificity, and odds ratios.
| Target Proteins | Epigallocate-3-Gallate (EGCG) | CBD | THC | Melanoxetin | Sitosterol |
|---|---|---|---|---|---|
| HER2 (ERBB2) | ↓ | ↓ | ↓ | ↓ | ↓ |
| Estrogen Receptor (ER) | ↑ | ↑ | ↑ | ↑ | ↑ |
| Progesterone Receptor (PR) | ↑ | ↑ | ↑ | ↑ | ↑ |
| Ki-67 | ↓ | ↓ | ↓ | ↓ | ↓ |
| P53 (TP53) | ↑ | ↑ | ↑ | ↑ | ↑ |
| BRCA1/BRCA2 | ↑ | ↑ | ↑ | ↑ | ↑ |
| Cyclin D1 | ↓ | ↓ | ↓ | ↓ | ↓ |
| E-cadherin | ↑ | ↑ | ↑ | ↑ | ↑ |
| VEGF | ↓ | ↓ | ↓ | ↓ | ↓ |
| MMP-9 | ↓ | ↓ | ↓ | ↓ | ↓ |
| CA15-3 | ↓ | ↓ | ↓ | ↓ | ↓ |
| CA27.29 | ↓ | ↓ | ↓ | ↓ | ↓ |
| EGFR | ↓ | ↓ | ↓ | ↓ | ↓ |
| HSP90 | ↓ | ↓ | ↓ | ↓ | ↓ |
| PD-L1 | ↓ | ↓ | ↓ | ↓ | ↓ |
| Cathepsin D | ↓ | ↓ | ↓ | ↓ | ↓ |
| Fibronectin | ↓ | ↓ | ↓ | ↓ | ↓ |
| S100A4 | ↓ | ↓ | ↓ | ↓ | ↓ |
| S100A7 | ↓ | ↓ | ↓ | ↓ | ↓ |
Note: Phytochemicals (EGCG, CBD, THC, etc.) show a downregulation of several key cancer biomarkers such as HER2, Ki-67, VEGF, and MMP-9, indicating potential for reducing tumor progression. P53, BRCA1/BRCA2, and E-cadherin show upregulation suggesting that these phytochemicals may help restore tumor-suppressive pathways. Standard drugs (Alendronate, Zoledronic Acid) show a general downregulation of biomarkers, though their effects are more limited compared to the phytochemicals in terms of restoring tumor-suppressive proteins.
Table 9. Pattern of serum protein expression levels in breast cancer rat model.
Therapeutic implications and future directions
The incorporation of phytochemicals into breast cancer management is novel since they are effective multitargeted agents, safe, and complementary to conventional treatments. These findings also need additional research on the interaction of EGCG and CBD with conventional chemotherapeutic agents with more therapeutic effects and fewer negative side effects. Further, complex in silico models and clinical trials are mandatory to confirm these findings and adjust the dosage regimens. Therefore, this work highlights phytochemicals as a place of hope for next-generation therapy against breast cancer because of the molecular details that it offers for the direction of personalized and precision medicine in oncology. This study demonstrates the significant potential of phytochemicals in breast cancer therapy, integrating docking studies, ADME analysis, and experimental data:
Binding affinities: Flavan-3-ols like EGCG (Epigallocatechin-3-Gallate), and cannabinoids like CBD and THC showed fairly high binding affinities to the important oncogenic proteins including VEGF, HER2, EGFR, etc.
Safety profiles: EGCG and melanoxetin were found to be a low risk; they offered little liver and gene toxicity.
Serum protein modulation: EGCG decreased both HER2 and VEGF protein expression thus implying antiproliferative/anti-angiogenic properties.
Based on these findings, phytochemicals are considered potential therapeutic agents of breast cancer; however, clinical application research and synergy work remain to be done. This work has shown that phytochemicals could be a source of effective drugs against breast cancer using the combination of molecular docking, ADME profiling, and experimental data. Polyphenols like EGCG and cannabinoids like CBD and THC revealed higher binding strengths with significant oncoprotein viz., VEGF, HER2, and EGFR. EGCG and melanoxetin have been shown to contain low levels of hepatotoxicity and mutagenicity. Furthermore, substantial inhibition of the nuclear expression of HER2 and VEGF by EGCG was demonstrated, thereby implying anti-proliferative and anti-angiogenic actions. These cumulative results endorse the potential use of phytochemicals for breast cancer treatment, although important clinical applications and combinational research still await phytochemicals’ translation.
Conclusion
Molecular analysis of phytochemical-protein interactions provided in this work suggests a novel approach to the treatment of breast cancer. Combining the docking studies and ADMET analysis with further experimental confirmation, it is shown that phytochemicals like EGCG, CBD, and THC have more versatile therapeutic activity in addressing important oncogenic proteins like VEGF, HER2, and EGFR. These observations convincingly argue for the oncogenic potential of phytochemicals and underscore their efficacy in inhibiting essential pathways causally associated with carcinogenesis, as well as their overall safety, which should not cause hepatotoxicity or mutagenicity. In addition, since EGCG changes serum protein concentration, specifically decreasing HER2 and VEGF having anti-proliferative and antiangiogenic action. Furthering its clinical application and combining it with other therapies, this study provides a rationale for the more effective utilization of phytochemicals in combating breast cancer. As the current discoveries about their molecular profiles strengthen, phytochemicals might turn out to be the main players in the future of targeted and individualized breast cancer treatments.
Acknowledgments
On behalf of all the authors would like to extend their deepest appreciation to all the participants who in one way or another have provided support for the completion of this research study. The authors would like to express their appreciation to the technical team of School of pain and Regenerative Medicine (SPRM), for the professional help they offered in experimental and computational information. We also thank Lab-313 management for allowing us access to important apparatus and materials during the study.
Author Contributions
Muhammad Akram Shahzad Khokhar participated in the experimental preparation, the acquisition of data, and the execution of biological assays. Arif Malik and Malik Ihsan Ullah Khan came up with the idea behind this study, oversaw the experimental part. Gul Zaib was in charge of the illustration of docking studies, ADME analysis, and final interpretation of the computational-based result. Statistical analysis and manuscript writing were done by Qurban Ali. Haleema Saadia critically reviewed the manuscript for important intellectual content and approved the final manuscript for publication.
Conflict of Interest
No conflicts of interest have been identified by the authors during the preparation of this manuscript.
Funding
None.
References
- Amin F, Hassan N, Khan F, Mohammad Z, Dilawar S, Bakhtiar M, Umer M, Mehmood K, Bashir K. (2024). Antimicrobial susceptibility profile of staphylococcus aureus isolated from salad samples. Bull Biol All Sci Res. 2024:88.
- Adinew GM, Messeha S, Taka E, Ahmed SA, Soliman KF. (2023). The role of apoptotic genes and protein-protein interactions in triple-negative breast cancer. Cancer Genomics Proteomics. 20:247-72.
[Crossref] [Google Scholar] [PubMed]
- Aggarwal V, Tuli HS, Tania M, Srivastava S, Ritzer EE, Pandey A, Aggarwal D, Barwal TS, Jain A, Kaur G, Sak K. (2022). Molecular mechanisms of action of epigallocatechin gallate in cancer: Recent trends and advancement. Semin Cancer Biol. 80:256-275.
[Crossref] [Google Scholar] [PubMed]
- Al-Ishaq RK, Overy AJ, Büsselberg D. (2020). Phytochemicals and gastrointestinal cancer: Cellular mechanisms and effects to change cancer progression. Biomolecules. 10:105.
[Crossref] [Google Scholar] [PubMed]
- Almatroodi SA, Alsahli MA, Aljohani ASM, Alhumaydhi FA, Babiker AY, Khan AA, Rahmani AH. (2022). Potential therapeutic targets of resveratrol, a plant polyphenol, and its role in the therapy of various types of cancer. Molecules. 27:2665.
[Crossref] [Google Scholar] [PubMed]
- Almatroodi SA, Alsahli MA, Rahmani AH. (2022). Berberine: An important emphasis on its anticancer effects through modulation of various cell signaling pathways. Molecules. 27:5889.
[Crossref] [Google Scholar] [PubMed]
- Almatroodi SA, Almatroudi A, Khan AA, Alhumaydhi FA, Alsahli MA, Rahmani AH. (2020). Potential therapeutic targets of epigallocatechin gallate (EGCG), the most abundant catechin in green tea, and its role in the therapy of various types of cancer. Molecules. 25:3146.
[Crossref] [Google Scholar] [PubMed]
- Almeida CF, Palmeira A, Valente MJ, Correia-da-Silva G, Vinggaard AM, Sousa ME, Teixeira N, Amaral C. (2024). Molecular targets of minor cannabinoids in breast cancer: In Silico and in vitro studies. Pharmaceuticals. 17:1245.
[Crossref] [Google Scholar] [PubMed]
- Awan SJ, Fatima Z, Kamran S, Khan AS, Fatima T, Imran S, Shabbir M, Nadeem SI. (2024). Guar gum in therapeutics: A succinct exploration. Bull Biol All Sci Res. 2024:60.
- Behroozaghdam M, Dehghani M, Zabolian A, Kamali D, Javanshir S, Hasani Sadi F, Hashemi M, Tabari T, Rashidi M, Mirzaei S, Zarepour A. (2022). Resveratrol in breast cancer treatment: From cellular effects to molecular mechanisms of action. Cell Mol Life Sci. 79:539.
[Crossref] [Google Scholar] [PubMed]
- Bhowmick S, AlFaris NA, ALTamimi JZ, ALOthman ZA, Aldayel TS, Wabaidur SM, Islam MA. (2020). Screening and analysis of bioactive food compounds for modulating the CDK2 protein for cell cycle arrest: Multi-cheminformatics approaches for anticancer therapeutics. J Mol Struct. 1216:128316.
- Çetinkaya M, Baran Y. (2023). Therapeutic potential of luteolin on cancer. Vaccines. 11:554.
[Crossref] [Google Scholar] [PubMed]
- Cherkasova V, Wang B, Gerasymchuk M, Fiselier A, Kovalchuk O, Kovalchuk I. (2022). Use of cannabis and cannabinoids for treatment of cancer. Cancers. 14:5142.
[Crossref] [Google Scholar] [PubMed]
- Chiu CF, Fu RH, Hsu SH, Yu YH, Yang SF, Tsao TC, Chang KB, Yeh CA, Tang CM, Huang SC, Hung HS. (2021). Delivery capacity and anticancer ability of the berberine-loaded gold nanoparticles to promote the apoptosis effect in breast cancer. Cancers. 13:5317.
[Crossref] [Google Scholar] [PubMed]
- Choudhari AS, Mandave PC, Deshpande M, Ranjekar P, Prakash O. (2020). Phytochemicals in cancer treatment: From preclinical studies to clinical practice. Front Pharmacol. 10:1614.
[Crossref] [Google Scholar] [PubMed]
- Choudhary MK, Pancholi B, Kumar M, Babu R, Garabadu D. (2025). A review on endoplasmic reticulum-dependent anti-breast cancer activity of herbal drugs: Possible challenges and opportunities. J Drug Target. 33:206-31.
[Crossref] [Google Scholar] [PubMed]
- Chunarkar-Patil P, Kaleem M, Mishra R, Ray S, Ahmad A, Verma D, Bhayye S, Dubey R, Singh HN, Kumar S. (2024). Anticancer drug discovery based on natural products: From computational approaches to clinical studies. Biomedicines. 12:201.
[Crossref] [Google Scholar] [PubMed]
- Costea T, Vlad OC, Miclea LC, Ganea C, SzöllÅsi J, Mocanu MM. (2020). Alleviation of multidrug resistance by flavonoid and non-flavonoid compounds in breast, lung, colorectal and prostate cancer. Int J Mol Sci. 21:401.
[Crossref] [Google Scholar] [PubMed]
- Davey MG, Hynes SO, Kerin MJ, Miller N, Lowery AJ. (2021). Ki-67 as a prognostic biomarker in invasive breast cancer. Cancers. 13:4455.
[Crossref] [Google Scholar] [PubMed]
- Desam NR, Al-Rajab AJ. (2022). Herbal biomolecules: Anticancer agents. Herbal Biomolecules in Healthcare Applications. 435-474.
- Eslami M, Memarsadeghi O, Davarpanah A, Arti A, Nayernia K, Behnam B. (2024). Overcoming chemotherapy resistance in metastatic cancer: A comprehensive review. Biomedicines. 12:183.
[Crossref] [Google Scholar] [PubMed]
- Gao Q, Feng J, Liu W, Wen C, Wu Y, Liao Q, Zou L, Sui X, Xie T, Zhang J, Hu Y. (2022). Opportunities and challenges for co-delivery nanomedicines based on combination of phytochemicals with chemotherapeutic drugs in cancer treatment. Adv Drug Deliv Rev. 188:114445.
[Crossref] [Google Scholar] [PubMed]
- Garg P, Awasthi S, Horne D, Salgia R, Singhal SS. (2023). The innate effects of plant secondary metabolites in preclusion of gynecologic cancers: Inflammatory response and therapeutic action. Biochim Biophys Acta Rev Cancer. 1878:188929.
[Crossref] [Google Scholar] [PubMed]
- George BP, Chandran R, Abrahamse H. (2021). Role of phytochemicals in cancer chemoprevention: Insights. Antioxidants. 10:1455.
[Crossref] [Google Scholar] [PubMed]
- Hossain A. (2021). Molecular docking, drug-likeness and ADMET analysis, application of Density Functional Theory (DFT) and molecular dynamics (MD) simulation to the phytochemicals from Withania somnifera as potential antagonists of Estrogen Receptor alpha (ER-α). Curr Comput Aided Drug Des. 17:797-805.
[Crossref] [Google Scholar] [PubMed]
- Kashif M, Nayer M, Shoukat A, Ullah A, Hassan N, Ullah J, SLIM M. (2024). Clinical validation of genexpert mtb/rif assay in the rapid diagnosis of extra pulmonary tuberculosis. Bull Biol All Sci Res. 2024:83.
- Kaur R, Bhardwaj A, Gupta S. (2023). Cancer treatment therapies: traditional to modern approaches to combat cancers. Mol Biol Rep. 50:9663-76.
[Crossref] [Google Scholar] [PubMed]
- Khan H, Labanca F, Ullah H, Hussain Y, Tzvetkov NT, Akkol EK, Milella L. (2022). Advances and challenges in cancer treatment and nutraceutical prevention: the possible role of dietary phenols in BRCA regulation. Phytochem Rev. 21:385-400.
- Khan MR, Trivedi V. (2024). Molecular modelling, docking and network analysis of phytochemicals from Haritaki churna: Role of protein cross-talks for their action. J Biomol Struct Dyn. 42:4297-312.
[Crossref] [Google Scholar] [PubMed]
- Kim ME, Kim DH, Lee JS. (2022). Transcription factors as targets of natural compounds in age-related diseases and cancer: Potential therapeutic applications. Int J Mol Sci. 23:13882.
[Crossref] [Google Scholar] [PubMed]
- Koh YC, Ho CT, Pan MH. (2020). Recent advances in cancer chemoprevention with phytochemicals. J Food Drug Anal. 28:14-37.
[Crossref] [Google Scholar] [PubMed]
- Kuran D, Pogorzelska A, Wiktorska K. (2020). Breast cancer prevention-is there a future for sulforaphane and its analogs? Nutrients. 12:1559.
[Crossref] [Google Scholar] [PubMed]
- Kurubanjerdjit N. (2020). Identifying the regulation mechanism of phytochemicals on triple negative breast cancer's biological network. Gene Rep. 19:100656.
- Lau KH, Tan AM, Shi Y. (2022). New and emerging targeted therapies for advanced breast cancer. Int J Mol Sci. 23:2288.
[Crossref] [Google Scholar] [PubMed]
- Luo Y, Yin S, Lu J, Zhou S, Shao Y, Bao X, Wang T, Qiu Y, Yu H. (2021). Tumor microenvironment: A prospective target of natural alkaloids for cancer treatment. Cancer Cell Int. 21:386.
[Crossref] [Google Scholar] [PubMed]
- Maiborodin I, Mansurova A, Chernyavskiy A, Romanov A, Voitcitctkii V, Kedrova A, Tarkhov A, Chernyshova A, Krasil’nikov S. (2022). Cancer angiogenesis and opportunity of influence on tumor by changing vascularization. J Pers Med. 12:327.
[Crossref] [Google Scholar] [PubMed]
- Malik A, Islam J, Zaib G, Ashraf MH, Zahid A, Rashid AR. (2024). Role of oxidative stress and immune response alterations in asthmatic pregnant females. Bull Biol All Sci Res. 2024:85.
- Malik A, Islam J, Zaib G, Saadia H, Zahid A. (2024). Correlation of oxidative stress markers in multiple biofluids of end-stage renal disease patients. Bull Biol All Sci Res. 2024:86.
- Marín V, Burgos V, Pérez R, Maria DA, Pardi P, Paz C. (2023). The potential role of epigallocatechin-3-gallate (EGCG) in breast cancer treatment. Int J Mol Sci. 24:10737.
[Crossref] [Google Scholar] [PubMed]
- MilivojeviÄ M, Pajic-LijakoviÄ I, DajiÄ Z, Dhara AK, Nayak AK, Hasnain MS. (2023). Nanotechnology in delivery and targeting of phytochemicals for lifestyle diseases. Role of Herbal Medicines. 2023:497-524.
- Mohapatra P, Singh P, Sahoo SK. (2020). Phytonanomedicine: A novel avenue to treat recurrent cancer by targeting cancer stem cells. Drug Discov Today. 25:1307-21.
[Crossref] [Google Scholar] [PubMed]
- Montané X, Kowalczyk O, Reig-Vano B, Bajek A, Roszkowski K, Tomczyk R, Pawliszak W, Giamberini M, Mocek-PÅóciniak A, Tylkowski B. (2020). Current perspectives of the applications of polyphenols and flavonoids in cancer therapy. Molecules. 25:3342.
[Crossref] [Google Scholar] [PubMed]
- Motallebi M, Bhia M, Rajani HF, Bhia I, Tabarraei H, Mohammadkhani N, Pereira-Silva M, Kasaii MS, Nouri-Majd S, Mueller AL, Veiga FJ. (2022). Naringenin: A potential flavonoid phytochemical for cancer therapy. Life Sci. 305:120752.
[Crossref] [Google Scholar] [PubMed]
- Munir S, Shah AA, Shahid M, Ahmed MS, Shahid A, Rajoka MS, Akash MS, Akram M, Khurshid M. (2020). Anti-angiogenesis potential of phytochemicals for the therapeutic management of tumors. Curr Pharm Des. 26:265-78.
[Crossref] [Google Scholar] [PubMed]
- Mustafa G, Younas S, Mahrosh HS, Albeshr MF, Bhat EA. (2023). Molecular docking and simulation-binding analysis of plant phytochemicals with the hepatocellular carcinoma targets epidermal growth factor receptor and caspase-9. Molecules. 28:3583.
[Crossref] [Google Scholar] [PubMed]
- Nahler G. (2024). Phytocannabinoids as chemotherapy adjuncts-a review for users. Onco. 4:287-321.
- Nawaz K, Khan S, Bibi A. (2024). Insights into scabies prevalence and risk factors. Bull Biol All Sci Res. 2024:68.
- Paul JK, Azmal M, Haque AS, Talukder OF, Meem M, Ghosh A. (2024). Phytochemical-mediated modulation of signaling pathways: A promising avenue for drug discovery. Advances in Redox Research. 13:100113.
- Prajapati KS, Shuaib M, Kushwaha PP, Singh AK, Kumar S. (2021). Identification of cancer stemness related miRNA (s) using integrated bioinformatics analysis and in vitro validation. 3 Biotech. 11:446.
[Crossref] [Google Scholar] [PubMed]
- Samad A, Waheed A, Shoukat A, Afridi R, Bibi A, Khan MI, Rabnawaz M, Rida T, Shah A, Zia T, Ullah J. (2024). Detection of clinical biomarkers associated with hepato and renal manifestations in covid-19 patients. Bull Biol All Sci Res. 2024:84.
- Shahiwala AF, Khan GA. (2023). Potential phytochemicals for prevention of familial breast cancer with BRCA mutations. Curr Drug Targets. 24:521-31.
[Crossref] [Google Scholar] [PubMed]
- Shekar N, Vuong P, Kaur P. (2024). Analysing potent biomarkers along phytochemicals for breast cancer therapy: An in silico approach. Breast Cancer Res Treat. 203:29-47.
[Crossref] [Google Scholar] [PubMed]
- Shrihastini V, Muthuramalingam P, Adarshan S, Sujitha M, Chen JT, Shin H, Ramesh M. (2021). Plant derived bioactive compounds, their anti-cancer effects and in silico approaches as an alternative target treatment strategy for breast cancer: An updated overview. Cancers. 13:6222.
[Crossref] [Google Scholar] [PubMed]
- Song T, Zhang H, Zhao Q, Hu Z, Wang Z, Song Y, Zhang Z. (2024). Small molecule inhibitor targeting the Hsp70-Bim protein–protein interaction in estrogen receptor-positive breast cancer overcomes tamoxifen resistance. Breast Cancer Res. 26:33.
[Crossref] [Google Scholar] [PubMed]
- Vaghasia H, Sakaria S, Prajapati J, Saraf M, Rawal RM. (2022). Interactive bioinformatics analysis for the screening of hub genes and molecular docking of phytochemicals present in kitchen spices to inhibit CDK1 in cervical cancer. Comput Biol Med. 149:105994.
[Crossref] [Google Scholar] [PubMed]
- van Geffen C, Heiss C, Deißler A, Kolahian S. (2022). Pharmacological modulation of myeloid-derived suppressor cells to dampen inflammation. Front Immunol. 13:933847.
[Crossref] [Google Scholar] [PubMed]
- Verhoog NJ, Spies LM. (2024). The anti-aromatase and anti-estrogenic activity of plant products in the treatment of estrogen receptor-positive breast cancer. J Steroid Biochem Mol Biol. 243:106581.
[Crossref] [Google Scholar] [PubMed]
- Vietri MT, D'elia G, Benincasa G, Ferraro G, Caliendo G, Nicoletti GF, Napoli C. (2021). DNA methylation and breast cancer: A way forward. Int J Oncol. 59:1-2.
[Crossref] [Google Scholar] [PubMed]
- Xu P, Yan F, Zhao Y, Chen X, Sun S, Wang Y, Ying L. (2020). Green tea polyphenol EGCG attenuates MDSCs-mediated immunosuppression through canonical and non-canonical pathways in a 4T1 murine breast cancer model. Nutrients. 12:1042.
[Crossref] [Google Scholar] [PubMed]
- Zhang Y, Li H, Zhang J, Zhao C, Lu S, Qiao J, Han M. (2020). The combinatory effects of natural products and chemotherapy drugs and their mechanisms in breast cancer treatment. Phytochem Rev. 19:1179-97.