Review Article - Modern Phytomorphology ( 2025) Volume 19, Issue 5
Impact of agonists of glucagon-like peptide-1 receptor in individuals with type 2 diabetes: A meta-analysis
Mohamed S. Imam1*, Fahad Saeed Algahtani2, Naif Ayidh Alshabab3, Manar Mesfer Eid Alsaadi4, Mastora Shajea Falaj Alotaibi4, Azoof Abdullah Naqi Alotaibi4, Abdulrahman Abdullah Zaben Alnefaie4, Retaj Awad AlNafie4, Raghad Khaled Alzahrani4, Sultan Ali A. Alqahtani4, Saeed Hussain Saeed Alzahrani5, Nawaf Mohammed A. Alhossan6, Latifa Fahad Abdalwahap Almohsin7, Ahmed Khalid Almutairi8 and Randa M. Abdel-Sattar92Department of Restorative Dental Science, Faculty of Dentistry, Taif University, P.O. Box 11099, Tai, Saudi Arabia
3College of Pharmacy, King Khalid University, Abha 62529, Saudi Arabia
4College of Pharmacy, Taif University, Taif 21944, Saudi Arabia
5College of Pharmacy, Umm Al-Qura University, Makkah, Saudi Arabia
6College of Pharmacy, Shaqra University, Shaqra 11961, Saudi Arabia
7College of Clinical Pharmacy, King Faisal University, Al-Ahsa, Eastern Province, Saudi Arabia
8Jamjoom Pharma Company, Riyadh, Saudi Arabia
9Department of Pharmaceutics, College of Pharmacy, Shaqra University, Shaqra 11961, Saudi Arabia
Mohamed S. Imam, Pharmacy Department, Alnahda College, Riyadh, Kingdom of Saudi Arabia, Email: imammohamed311@gmail.com
Received: 13-May-2025, Manuscript No. mp-25-165485; Accepted: 25-Nov-2025, Pre QC No. mp-25-165485 (PQ); Editor assigned: 15-May-2025, Pre QC No. mp-25-165485 (PQ); Reviewed: 18-Sep-2025, QC No. mp-25-165485; Revised: 24-Nov-2025, Manuscript No. mp-25-165485 (R); Published: 02-Dec-2025, DOI: 10.5281/zenodo.17906205
Abstract
Background and objectives: We have done a meta-analysis to assess influence of glucagon-like peptide-1 receptor agonists (GLP1RAs) in individuals with Type 2 Diabetes Mellitus (T2DM). Beyond clinical relevance, GLP1RAs act through metabolic pathways that may also be influenced by plant-derived bioactive compounds, suggesting possible phytotherapeutic implications.
Materials and methods: A thorough literature search conducted till January 2025 identified 10 studies encompassing 1,639,510 patients with T2DM at study’s inception. Study reported correlations between effects of Impact of GLP1RAs in individuals with T2DM. We computed Odds Ratio (OR) with 95% Confidence Intervals (CIs) to evaluate impact of Impact of GLP1RAs in individuals with T2DM, employing dichotomous method with either a random or fixed-effect model.
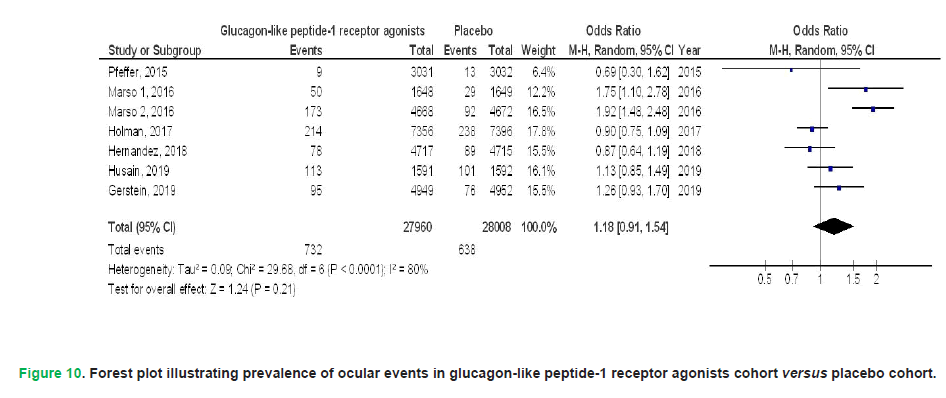
Results: GLP1RAs demonstrated significantly reduced all-cause mortality (OR, 0.77; 95% CI, 0.66-0.90, p<0.001), cardiovascular mortality (OR, 0.87; 95% CI, 0.81-0.94, p<0.001), myocardial infarction (OR, 0.92; 95% CI, 0.85-0.98, p=0.01), stroke (OR, 0.87; 95% CI, 0.83-0.91, p<0.001), hospital admissions owing to heart failure (OR, 0.86; 95% CI, 0.74-1.00, p=0.05), and renal events (OR, 0.83; 95% CI, 0.77-0.89, p<0.001), as well as a composite kidney outcome including macroalbuminuria (OR, 0.86; 95% CI, 0.83-0.89, p<0.001) and deterioration of kidney function (OR, 0.83; 95% CI, 0.74-0.93, p=0.002) when compared to placebo in individuals with T2DM. No significant difference was detected between GLP1RAs and placebo regarding ocular events (OR, 1.18; 95% CI, 0.91-1.54, p=0.21) and
three-component major adverse cardiovascular events (OR, 0.99; 95% CI, 0.65-1.51, p=0.95) in individuals with T2DM.
Conclusion: GLP1RAs were associated with significantly reduced all-cause mortality, cardiovascular mortality, myocardial infarction, stroke, hospital admissions for heart failure, and renal events, as well as a composite kidney outcome including macroalbuminuria and deterioration of kidney function. Yet, no significant differences were detected in ocular events and three-component major adverse cardiovascular events when compared to placebo in individuals with T2DM. Importantly, these findings highlight incretin-based mechanisms as potential targets for plant-derived compounds (e.g., flavonoids, alkaloids, and terpenoids) that can modulate glucose homeostasis. Future research should integrate phytochemical screening and phytomorphological studies to identify natural agents with
GLP1RA-like activity.
Keywords
Type 2 diabetes mellitus, Cardiovascular, Glucagon-like peptide-1 receptor agonists, Mortality, Eye, Kidney, Phytochemicals, Plant-derived bioactive compounds, Flavonoids, Phytotherapy, Gymnema sylvestre, Momordica charantia
Introduction
Effectively reducing the occurrence of cardiovascular events stands as a cornerstone in the management of individuals diagnosed with Type 2 Diabetes Mellitus (T2DM) (Zelniker, et al. 2019). Beyond conventional treatments focused on blood pressure and cholesterol control, Glucagon-Like Peptide-1 Receptor Agonists (GLP1RAs) and sodium-glucose cotransporter-2 inhibitors have emerged as therapeutic agents demonstrating a notable capacity to lower cardiovascular risk. GLP1RAs exert their antihyperglycemic effects by enhancing insulin release in a glucose-dependent manner while simultaneously suppressing glucagon secretion. Furthermore, they contribute to slower gastric emptying and reduced appetite (Das, et al. 2018, Davies, et al. 2018). Notably, the use of GLP1RAs in managing T2DM is associated with a low risk of hypoglycemia and offers the additional benefits of modest improvements in lipid profiles, reductions in blood pressure levels, and a decrease in body weight. Although, medications in this class range in their construction and period of action, they were examined using various sample sizes and variable groups (Baggio and Drucker, 2007, Marre, et al. 2009). Impact of GLP1RAs on cardiovascular system in those clinical trials is inconsistent.
In parallel, numerous plant-derived compounds such as flavonoids, alkaloids, and terpenoids have been reported to modulate glucose metabolism, insulin sensitivity, and even GLP-1 secretion, highlighting a potential link between pharmacological GLP1RA action and phytotherapy. Integrating insights from both clinical and phytochemical studies can help identify novel plant-based agents acting on incretin pathways. This study aims to conduct a meta-analysis to assess influence of GLP1RAs in individuals with T2DM. Several medicinal plants, including Gymnema sylvestre (gurmar), Momordica charantia (bitter melon), and Cinnamomum verum (true cinnamon), have been investigated for antidiabetic effects and may influence incretin biology.
Materials and Methods
This study adhered to meta-analysis of epidemiological research as outlined in statement, conducted according to a predetermined process (Stroup, et al. 2000).
Study selection
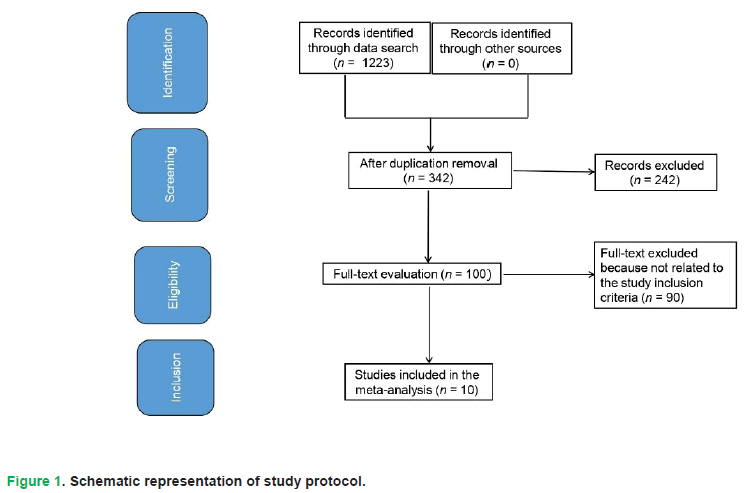
Included studies utilized statistical to compare effects of Impact of GLP1RAs in individuals with T2DM (Emad, et al. 2023). Studies eligible for inclusion were restricted to those involving human participants and published in English. No constraints were applied to study scale or methodological design during the selection process. Exclusion criteria targeted non-empirical works, such as reviews and opinion pieces, as well as investigations lacking quantitative outcome measures. A comprehensive visualization of the study selection protocol is provided in Fig. 1.
Figure 1: Schematic representation of study protocol.
Eligible studies met the following predefined criteria:
- Study design: Prospective Randomized Controlled Trials (RCTs) or retrospective cohort analyses.
- Population: Participants with a confirmed diagnosis of T2DM.
- Intervention: Therapeutic protocols involving GLP1RAs.
- Comparator: Direct comparisons between GLP1RA regimens and placebo controls.
Criteria for excluding participants from intervention groups were:
- Research that failed to ascertain impact of Impact of GLP1RAs in individuals with T2DM.
- Research on diabetes mellitus treatments excluding GLP1Ras.
- Research did not concentrate on impact on comparative outcomes.
Identification
To methodically evaluate the therapeutic effects of GLP1RAs in patients with T2DM, a structured literature search was designed and executed in alignment with the PICOS (Population, Intervention, Comparator, Outcomes, Study Design) framework (Higgins, et al. 2003, Liberati, et al. 2009). This framework provided a structured approach, defining the Population as individuals diagnosed with T2DM, the Intervention/Exposure as treatment with GLP1RAs, the Comparison as GLP1RAs versus placebo, the Outcome as changes in the prevalence of various health outcomes in individuals with T2DM, and the Study design as having no restrictions. Subsequently, an exhaustive systematic search was conducted across several major databases, including PubMed, Embase, OVID, Google Scholar, and the Cochrane Library, covering the literature up to January 2025. This search employed a carefully constructed combination of keywords and related terms specifically focused on GLP1RAs, T2DM, mortality, cardiovascular health, renal function, and ocular conditions, with the precise search terms detailed in Tab. 1.
| Database | Search Strategy |
|---|---|
| Pubmed | #1 «glucagon-like peptide-1 receptor agonists»[MeSH Terms] OR «type 2 diabetes mellitus»[All Fields] OR «mortality»[All Fields] #2 «cardiovascular»[MeSH Terms] OR «glucagon-like peptide-1 receptor agonists»[All Fields] OR «kidney»[All Fields] OR «eye»[All Fields] #3 #1 AND #2 |
| Embase | ‘glucagon-like peptide-1 receptor agonists’/exp OR ‘type 2 diabetes mellitus’/exp OR ‘mortality’/exp #2 ‘cardiovascular’/exp OR ‘ICBG’/exp OR ‘kidney’/exp OR ‘eye’/exp #3 #1 AND #2 |
| Cochrane library | #1 (glucagon-like peptide-1 receptor agonists):ti,ab,kw OR (type 2 diabetes mellitus):ti,ab,kw OR (mortality):ti,ab,kw (Word variations have been searched) #2 (cardiovascular):ti,ab,kw OR (kidney):ti,ab,kw OR (eye):ti,ab,kw (Word variations have been searched) #3 #1 AND #2 |
Table 1. Search strategy for each database.
All the studies identified through this process were initially compiled into an endnote library. Following this, duplicate entries were removed, and the titles and abstracts of the remaining studies were meticulously reviewed to exclude those that did not demonstrate any connection between the effects of GLP1RAs and individuals with T2DM. The studies that met the inclusion criteria then underwent a thorough analysis to extract relevant information.
Screening
To ensure consistency and facilitate analysis, data from the included studies were systematically organized into a standardized format based on characteristics related to the study itself and the participants involved (Gupta, et al. 2018). For each included trial, epidemiological parameters such as the lead investigator’s surname, study timeframe (including publication year), and geographic location (country and region) were systematically extracted, alongside details on methodological design. Information about the study population included the type of individuals participating, the total number of subjects, their demographic characteristics, as well as relevant clinical features and management approaches.
Following predefined inclusion criteria, two independent researchers extracted the data. Any disagreements that arose during this process were resolved by consulting the corresponding author of the original study to reach a consensus. In cases where a single clinical trial evaluated the effects of GLP1RAs in individuals with T2DM and presented distinct sets of data, each of these individual data points was extracted separately.
To ensure the robustness of synthesized evidence, two investigators independently appraised the risk of bias across included studies. Methodological rigor was evaluated using the Cochrane RoB 2 tool, a validated framework for assessing bias in randomized controlled trials (RoB, 2020). Based on predefined criteria, studies were categorized into three tiers:
1. Low risk: Full adherence to all methodological standards.
2. Moderate risk: Partial compliance or ambiguous reporting of key criteria.
3. High risk: Critical methodological omissions or deviations.
Discrepancies in bias ratings were resolved through consensus-driven reappraisal of primary source materials (Sayed, et al. 2023).
Eligibility
Primary outcome focused on impact of GLP1RAs in individuals with T2DM. An evaluation of effects of GLP1RAs compared to placebo on these outcomes in subjects with T2DM was compiled into a summary.
Inclusion
Sensitivity analyses were confined to studies that reported effects of Impact of GLP1RAs in individuals with T2DM. For sensitivity analysis and subcategory we compared GLP1RAs with placebo.
Statistical analysis
To aggregate quantitative evidence, pooled Odds Ratios (ORs) and 95% Confidence Intervals (CIs) were derived through meta- analytical frameworks. Data synthesis was performed using either binary or continuous variable models, with selection between random-effects or fixed-effects approaches guided by observed heterogeneity levels. Interstudy variability was quantified via the I² statistic, a standardized metric (range: 0-100%) reflecting the proportion of total variability attributable to true differences rather than chance.
According to established conventions (Higgins, et al. 2003), I² values were interpreted as indicating no (around 0%), low (around 25%), moderate (around 50%), or significant (around 75%) heterogeneity. In our meta-analysis, we adopted a decision rule whereby a random-effects model was applied when the I² value exceeded 50%, indicating substantial heterogeneity, while a fixed-effects model was used when the I² value was below 50%, suggesting more homogeneity across the studies. To further explore potential sources of variability and to examine the consistency of findings across different contexts, we performed subgroup analyses by stratifying the initial evaluations based on predefined outcome categories, as described in our earlier methodology. A p-value of less than 0.05 was set as the threshold for determining statistically significant differences between these subgroups. From a phytotherapy perspective, subgroup or sensitivity analyses could also investigate whether plant-based compounds targeting GLP-1 or related metabolic pathways yield comparable cardiovascular, renal, or metabolic outcomes. This would provide a bridge between pharmacological and phytochemical evidence. Future subgroup analyses might also stratify findings by synthetic versus plant-derived GLP-1 modulators, enabling comparative insights into efficacy and safety profiles.
To evaluate the potential for publication bias, we employed both objective and subjective methods. Objectively, we conducted Egger’s regression test, where a p-value greater than or equal to 0.05 was considered indicative of significant bias. Subjectively, we visually inspected funnel plots, which graph the logarithm of the odds ratios against their standard errors, to assess the symmetry of the plot, with asymmetry suggesting possible publication bias (Gupta, et al. 2018). All hypothesis testing employed two-tailed significance thresholds, with statistical significance defined a priori as p<0.05 for all analytical endpoints unless explicitly stated otherwise. Statistical analyses and visual outputs, including forest plots and funnel plots, were generated using Review Manager 5.3 (The Nordic Cochrane Centre, Cochrane Collaboration, Copenhagen, Denmark), a validated software platform for meta-analytical computations.
Results
A total of 1,223 distinct research articles were identified, of which 10 investigations (conducted between 2015 and 2024) met inclusion criteria and were incorporated into analysis (Pfeffer, et al. 2015, Marso, Bain, et al. 2016, Marso, Daniels, et al. 2016, Holman, et al. 2017, Hernandez, et al. 2018, Gerstein, et al. 2019, Husain, et al. 2019, Huang, et al. 2021, Elsaid, et al. 2024, Yen, et al. 2024).
The ten studies encompassed 1,639,510 participants diagnosed with T2DM at outset; 60,651 were administered GLP1RAs, while 1,576,859 received a placebo. All studies assessed impact of GLP1RAs versus placebo on mortality, cardiovascular health, renal function, and ocular outcomes in individuals with T2DM.
The study population varied from 3,183 to 1,569,553 participants with T2DM at commencement of research. Specifics of ten investigations are presented in Tab. 2.
| Study | Country | Total | Glucagon-like peptide-1 receptor agonists | Placebo |
|---|---|---|---|---|
| Pfeffer, et al. (2015) | USA and Europe | 6068 | 3034 | 3034 |
| Marso, et al. (2016) | USA and Europe | 3297 | 1648 | 1649 |
| Marso, et al. (2016) | USA | 9340 | 4668 | 4672 |
| Holman, et al. (2017) | UK | 14752 | 7356 | 7396 |
| Hernandez, et al. (2018) | USA and Europe | 9463 | 4731 | 4732 |
| Gerstein, et al. (2019) | USA and Europe | 9901 | 4949 | 4952 |
| Husain, et al. (2019) | USA and Europe | 3183 | 1591 | 1592 |
| Huang, et al. (2021) | Taiwan | 5657 | 1057 | 4600 |
| Elsaid, et al. (2024) | USA | 6296 | 401 | 5895 |
| Yen, et al. (2024) | China | 1569553 | 31216 | 1538337 |
| Total | 1637510 | 60651 | 1576859 |
Table 2. Characteristics of selected studies for meta-analysis.
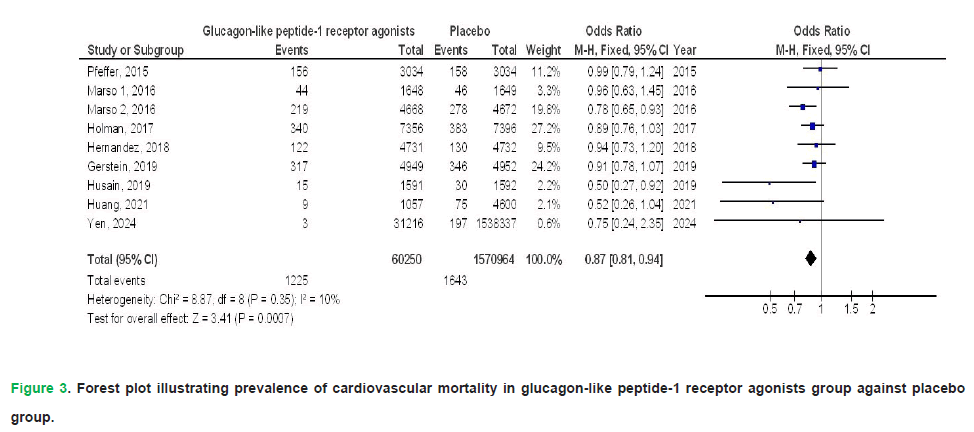
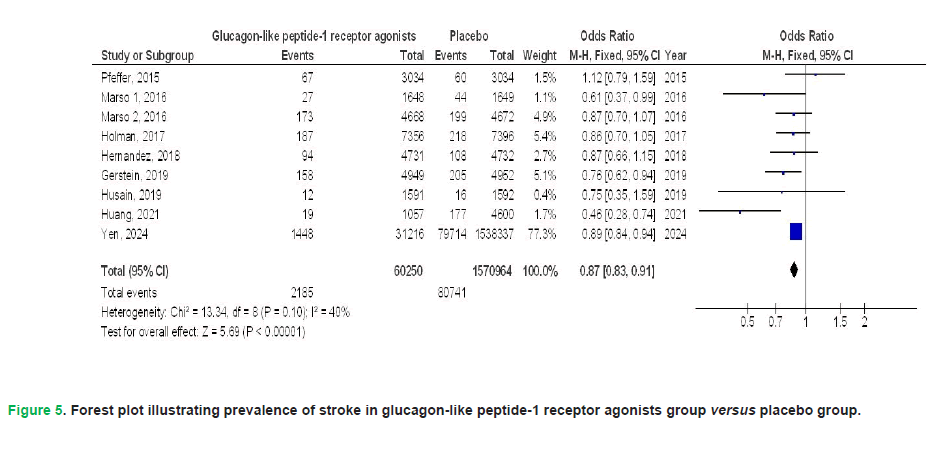
In individuals with T2DM, the pooled meta-analysis revealed that GLP1RAs demonstrated significant benefits compared to placebo across several key outcomes. Specifically, GLP1RAs were associated with a statistically significant reduction in the risk of all-cause mortality (Odds Ratio [OR], 0.77; 95% Confidence Interval [CI], 0.66-0.90; p<0.001), although this finding exhibited considerable heterogeneity (I²=79%). The therapeutic use of GLP1RAs demonstrated a significant reduction in cardiovascular mortality risk (OR: 0.87; 95% CI: 0.81-0.94; p<0.001), with negligible heterogeneity (I²=10%). Similarly, GLP1RA therapy was associated with lower incidence rates of myocardial infarction (OR: 0.92; 95% CI: 0.85-0.98; p=0.01; I²=42%) and stroke (OR: 0.87; 95% CI: 0.83-0.91; p<0.001; I²=40%), both outcomes exhibiting low interstudy variability.
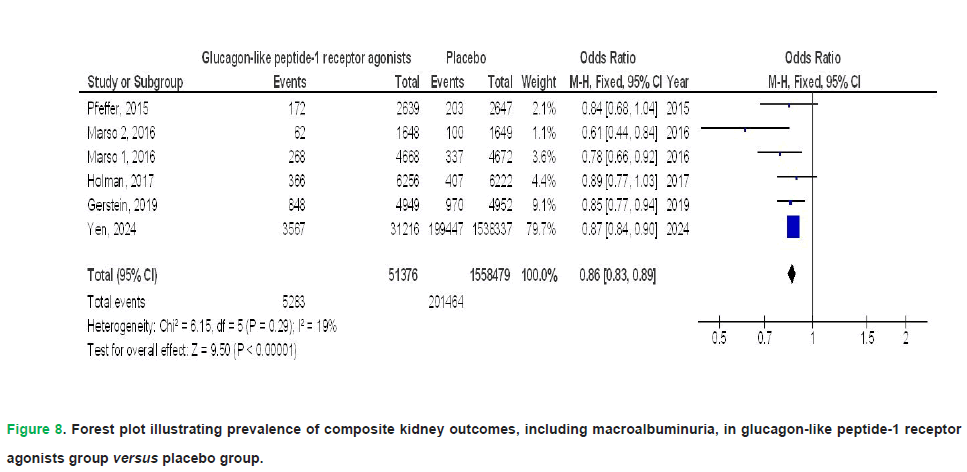
GLP1RA therapy demonstrated a trend toward reduced hospitalization due to heart failure (OR: 0.86; 95% CI: 0.74-1.00; p=0.05), though with substantial between-study variability (I²=76%). Concurrently, it exhibited a statistically significant decline in renal complications (OR: 0.83; 95% CI: 0.77-0.89; p<0.001), with no observed heterogeneity across trials (I²=0%). Regarding kidney-related outcomes, GLP1RAs were associated with a lower incidence of the composite kidney outcome, including macroalbuminuria (OR, 0.86; 95% CI, 0.83-0.89; p<0.001), which showed no heterogeneity (I²=19%), and a reduced risk of worsening kidney function (OR, 0.83; 95% CI, 0.74-0.93; p=0.002), also with no significant heterogeneity (I²=22%). These findings are visually represented in Figs. 2-9.
Figure 2: Forest plot illustrating prevalence of all-cause mortality in glucagon-like peptide-1 receptor agonists group versus placebo group.
Figure 3: Forest plot illustrating prevalence of cardiovascular mortality in glucagon-like peptide-1 receptor agonists group against placebo group.
Figure 4: Forest plot illustrating prevalence of myocardial infarction in glucagon-like peptide-1 receptor agonists cohort against placebo cohort.
Figure 5: Forest plot illustrating prevalence of stroke in glucagon-like peptide-1 receptor agonists group versus placebo group.
Figure 6: Forest plot illustrating prevalence of hospital admissions for heart failure in glucagon-like peptide-1 receptor agonists group versus placebo group.
Figure 7: Forest plot illustrating prevalence of renal events in glucagon-like peptide-1 receptor agonists cohort versus placebo cohort.
Figure 8: Forest plot illustrating prevalence of composite kidney outcomes, including macroalbuminuria, in glucagon-like peptide-1 receptor agonists group versus placebo group.
Figure 9: Forest plot illustrating prevalence of deteriorating kidney function in glucagon-like peptide-1 receptor agonists group against placebo group.
Pooled analyses revealed no statistically meaningful disparity between GLP1RAs and placebo in the incidence of ocular complications (OR: 1.18; 95% CI: 0.91-1.54; p=0.21), marked by substantial between-study variability (I²=80%), or in the three-component MACE endpoint (OR: 0.99; 95% CI: 0.65-1.51; p=0.95), characterized by extreme heterogeneity (I²=99%), among patients with T2DM. These findings are visually summarized in Figs 10 and 11. Selected studies conducted stratified analyses that did not account for individual-level characteristics or ethnicity, as no research provided or adjusted for these variables. The integrity of the synthesized evidence was reinforced by the absence of detectable publication bias. This conclusion was drawn from a symmetrical funnel plot upon graphical evaluation, which showed no significant asymmetry, and corroborated statistically by Egger’s linear regression analysis (p=0.89). Additionally, the majority of incorporated studies demonstrated robust methodological rigor, attributable to their retrospective or prospective observational frameworks and the inclusion of extensive participant cohorts, enhancing the reliability of pooled estimates. Furthermore, a thorough evaluation of the included studies revealed no indication of selective reporting bias, as all articles provided comprehensive outcome data without any apparent omission or preferential reporting of specific results. Although the current findings are based on synthetic GLP1RA therapies, future analyses could investigate whether plant-derived compounds with GLP-1 receptor activity demonstrate comparable outcomes in cardiovascular, renal, and metabolic domains.
Figure 10: Forest plot illustrating prevalence of ocular events in glucagon-like peptide-1 receptor agonists cohort versus placebo cohort.
Figure 11: Forest plot illustrating prevalence of three-component major adverse cardiovascular events in glucagon-like peptide-1 receptor agonists group versus placebo group.
Discussion
This meta-analysis, encompassing 10 studies, involved 1,639,510 patients with T2DM at baseline; 60,651 received GLP1RAs, while 1,576,859 were assigned to placebo. GLP1RAs demonstrated marked reductions in all-cause and cardiovascular mortality, alongside lower incidence rates of myocardial infarction, stroke, and heart failure-related hospitalizations in patients with T2DM compared to placebo. Additionally, GLP1RA therapy was linked to improved renal outcomes, including decreased risks of renal events, composite kidney endpoints (e.g., macroalbuminuria progression), and slower deterioration of renal function.
Comparative analyses revealed no statistically meaningful disparities between GLP1RAs and placebo in the incidence of ophthalmic complications or three-component Major Adverse Cardiovascular Events (MACE) among individuals with T2DM (Pfeffer, et al. 2015, Marso, Bain, et al. 2016, Marso, Daniels, et al. 2016, Holman, et al. 2017, Hernandez, et al. 2018, Gerstein, et al. 2019, Husain, et al. 2019, Huang, et al. 2021, Elsaid, et al. 2024, Yen, et al. 2024). This is the first meta-analysis to underscore adverse ocular occurrences in people utilizing GLP1RAs in comparison to placebo. No prior meta-analyses have reported this effect (Zelniker, et al. 2019, Duan, et al. 2019, Marsico, et al. 2020, Men, et al. 2020, Alfayez, et al. 2020, Kristensen, et al. 2019). Recent trials yield incongruent findings about the association between GLP1RAs and diabetic retinopathy (Douros, et al. 2018, Gaborit, et al. 2020). Given the limited number of studies incorporated into our meta-analysis, interpretation of the observed outcomes should be approached with caution. This constraint underscores the importance of further research to either substantiate these initial findings or, conversely, to significantly alter the confidence in our assessment of the treatment’s influence. The AMPLITUDE-O trial, a cardiovascular outcomes study currently underway, is investigating efpeglenatide a long-acting Glucagon-Like Peptide-1 Receptor Agonist (GLP1RA) derived from exendin-4. This trial is poised to deliver pivotal insights that may refine clinical understanding of cardiovascular risk modulation in T2DM populations (Gaborit, et al. 2021). The results of this trial are eagerly awaited as they may offer more definitive insights and potentially strengthen or refine the conclusions drawn from the current body of evidence. Liraglutide, albiglutide, semaglutide, and dulaglutide resemble GLP1, whereas exenatide and lixisenatide are derived from exendin-4. Period of action varies among these agents (Madsbad, 2016, Lund, et al. 2014). Although duration did not alter treatment effect, a probable interaction linked to chemical structure was suggested, with possible implications for main adverse cardiovascular events associated with exendin-4 drugs, while this finding did not achieve statistical significance (Kristensen, et al. 2019).
Our results may not sufficiently challenge guideline recommendations advocating use of GLP1RAs to mitigate cardiovascular event risk in individuals with cardiovascular disease, although they provide additional data backing up these commendations (Das, et al. 2018, Davies, et al. 2018). Variations in the length of the observation period across different clinical trials might explain some of the inconsistencies seen in their results. For instance, the ELIXA trial (Pfeffer, et al. 2015) had a shorter median follow-up duration of 2.1 years in comparison to the LEADER trial (Marso, Daniels, et al. 2016), which followed participants for a median of 3.8 years. However, our meta-analysis suggests that the duration of follow-up did not appear to significantly modify the beneficial effects of GLP1RAs on cardiovascular event outcomes. Notably, the positive impact of GLP1RA treatment on cardiovascular events remained consistent across various subgroups defined by age and renal function (Pfeffer, et al. 2015, Marso, Bain, et al. 2016, Marso, Daniels, et al. 2016, Holman, et al. 2017, Hernandez, et al. 2018, Gerstein, et al. 2019, Husain, et al. 2019, Huang, et al. 2021). It is anticipated that the absolute benefit derived from GLP1RA therapy could be more pronounced in these specific subgroups, given that older age and reduced estimated glomerular filtration rate are independently associated with a higher incidence of MACE (Zelniker, et al. 2019, Duan, et al. 2019, Marsico, et al. 2020, Men, et al. 2020, Alfayez, et al. 2020, Kristensen, et al. 2019). In clinical management, GLP1RAs may serve as a viable alter native for patients with heart failure or renal impairment who are intolerant to Sodium-Glucose Co-Transporter-2 inhibitors (SGLT2i), offering a tailored pharmacological strategy to address cardiometabolic comorbidities (Zelniker, et al. 2019).
The mechanistic basis for the cardioprotective effects of GLP1RAs remains incompletely understood, particularly given their lack of demonstrable clinical benefit in trials involving patients with Heart Failure and reduced Ejection Fraction (HFrEF) (Margulies, et al. 2016, Jorsal, et al. 2017). The observed benefit in the meta-analysis appears mechanistically driven by a decreased incidence of Myocardial Infarction (MI), a condition that most commonly precedes the development of Heart Failure (HF) in clinical trajectories (Pfeffer, et al. 2015 del Olmo-Garcia, et al. 2018). This idea necessitates additional research, including examining temporal sequence of cardiovascular events in individuals with T2DM. GLP1RAs are considered cardioprotective agents (Dalsgaard, et al. 2018, Read, et al. 2012). Certain investigations indicated that GLP1RAs possess an anti-atherothrombotic effect (Dalsgaard, et al. 2018, Read, et al. 2012). This benefit contrasts with SGLT2i, which demonstrate a faster onset of cardiovascular benefits and greater efficacy in mitigating heart failure progression, implying that combination therapy with both drug classes could yield additive or synergistic therapeutic effects (Zelniker, et al. 2019). Consequently, cardiovascular impact of GLP1RAs may account for reduced hospital admissions for heart failure, renal complications, and decreased mortality observed in our meta-analysis.
This meta-analysis revealed distinct comparative effects of GLP1RAs versus placebo on mortality rates, cardiovascular outcomes, renal endpoints, and ophthalmic complications in patients diagnosed with T2DM. Nevertheless, further studies are required both to corroborate these associations and to determine whether the observed effects translate into clinically significant outcomes. This was also indicated in other analogous meta-analyses, which demonstrated a comparable influence of GLP1RAs and placebo in individuals with T2DM (Zelniker, et al. 2019, Duan, et al. 2019, Marsico, et al. 2020, Men, et al. 2020, Alfayez, et al. 2020, Kristensen, et al. 2019). The equivocal findings associated with GLP1RAs in this meta-analysis warrant further investigation, as no conclusive mechanistic explanation emerged to account for these observations. Future studies should rigorously examine the interplay of demographic variables (e.g., ethnicity) and patient-level covariates, given that this analysis failed to establish definitive correlations between these factors and clinical outcomes. Future large-scale randomized controlled trials and patient-level meta-analyses will be essential to clarify the role of GLP1RAs in diverse populations and to establish whether these agents should be preferentially recommended for patients with specific comorbidities. These clinical results motivate targeted phytomorphological screening of species such as Gymnema sylvestre, Momordica charantia, and Berberis aristata for bioactive molecules that may mimic or modulate GLP-1 pathways (Tiwari, et al. 2014, Kumar, et al. 2018, Rao, et al. 2014, Singh, et al. 2014, Abiola, et al. 2024).
Limitations
This meta-analysis may be subject to selection bias, as several identified studies were excluded because they did not align with our predefined inclusion criteria. Additionally, we could not ascertain the correlation of the observed results with participant-level data or demographic variables such as ethnicity, which represents a limitation in our understanding of the findings’ broader applicability. This investigation aimed to assess the comparative therapeutic efficacy of GLP1RAs versus placebo in patients with T2DM, leveraging pooled datasets from prior clinical trials. This approach inherently carries the potential for bias due to limitations in the information reported in the original publications. It should be noted that all studies included in this meta-analysis were observational in nature, and a considerable degree of heterogeneity was observed across the included publications. Several factors could have contributed to this heterogeneity and introduced bias, such as variations in patient compliance, the absence of detailed subject-level data, differences in the ethnicity and nutritional health of the participants across studies. Finally, the potential for publication bias due to unpublished studies and missing data in the aggregated results cannot be entirely ruled out. Participants utilized varying treatment regimens, dosages, and healthcare systems.
Conclusion
In individuals with T2DM, GLP1RAs were associated with significant reductions in all-cause mortality, cardiovascular mortality, myocardial infarction, stroke, hospitalizations for heart failure, renal events, composite kidney outcomes including macroalbuminuria, and deterioration of kidney function compared to placebo. However, no significant differences observed between GLP1RAs and in relation to ocular events or the three-component Major Adverse Cardiovascular Events (MACE). Further research is required to corroborate these findings. Interpretation of the outcomes should be approached with caution, given the limited number of studies included in this meta-analysis. Additional investigations are needed to validate these results and to strengthen confidence in the evaluation of treatment effects. From a phytomorphological perspective, these findings also encourage exploration of plant-derived bioactive compounds with structural or functional similarities to GLP1RAs, as plants remain a rich source of peptides and secondary metabolites with potential antidiabetic and cardioprotective effects. This integrative approach may expand the scope of GLP1RA-related therapies toward naturally inspired molecules.
Author Contributions
Conceptualization: Mohamed S. Imam, Randa M. Abdel-Sattar and Abdulrahman Abdullah Zaben Alnefaie; Methodology: Mo- hamed S. Imam and Saeed Hussain Saeed Alzahrani; Software: Mohamed S. Imam and Raghad Khaled Alzahrani; Validation: Mo- hamed S. Imam and Abdulrahman Abdullah Zaben Alnefaie; Formal analysis: Mohamed S. Imam and Nawaf Mohammed A. Alhossan; Investigation: Mohamed S. Imam; Resources: Manar Mesfer Eid Alsaadi; Data curation: Retaj Awad AlNafie and Latifa Fahad Abdal- wahap Almohsin; Writing-original draft preparation: Mohamed S. Imam, Fahad Saeed Algahtani, Naif Ayidh Alshabab, Abdulrahman Abdullah Zaben Alnefaie, Manar Mesfer Eid Alsaadi, Sultan Ali A. Alqahtani, Retaj Awad AlNafie; Writing-review and editing: Mohamed S. Imam, Raghad Khaled Alzahrani, Saeed Hussain Saeed Alzahrani, Nawaf Mohammed A. Alhossan, Latifa Fahad Abdalwahap Almohsin, Randa M. Abdel-Sattar, Mastora Shajea Falaj Alotaibi, Azoof Abdullah Naqi Alotaibi, Ahmed Khalid Almutairi; Visualization: Mohamed S. Imam, Mastora Shajea Falaj Alotaibi and Azoof Abdullah Naqi Alotaibi; Supervision: Mohamed S. Imam; Project administration: Mohamed S. Imam; Funding acquisition: Mohamed S. Imam. All authors have read and agreed to the published version of the manuscript.Funding
This research received no external funding.Conflicts of Interests
The authors declare no conflict of interest.
References
- Abiola JO, Oluyemi AA, Idowu OT, Oyinloye OM, Ubah CS, Owolabi OV, Somade OT, Onikanni SA, Ajiboye BO, Osunsanmi FO, Nash O. (2024). Potential role of phytochemicals as glucagon-like peptide 1 receptor (GLP-1R) agonists in the treatment of diabetes mellitus. Pharmaceuticals. 17:736.
- Alfayez OM, Almohammed OA, Alkhezi OS, Almutairi AR, Al Yami MS. (2020). Indirect comparison of glucagon like peptide-1 receptor agonists regarding cardiovascular safety and mortality in patients with type 2 diabetes mellitus: Network meta-analysis. Cardiovasc Diabetol. 19:96.
- Baggio LL, Drucker DJ. (2007). Drucker, biology of incretins: GLP-1 and GIP. Gastroenterology. 132:2131-2157.
- Dalsgaard NB, Vilsbøll T, Knop FK. (2018). Effects of glucagon like peptide 1 receptor agonists on cardiovascular risk factors: A narrative review of head to head comparisons. Diabetes Obes Metab. 20:508-519.
- Das SR, Everett BM, Birtcher KK, Brown JM, Cefalu WT, Januzzi JL, Kalyani RR, Kosiborod M, Magwire ML, Morris PB, Sperling LS. (2018). 2018 ACC expert consensus decision pathway on novel therapies for cardiovascular risk reduction in patients with type 2 diabetes and atherosclerotic cardio- vascular disease: A report of the American college of cardiology task force on expert consensus decision pathways. J Am Coll Cardiol. 72:3200-3223.
- Davies MJ, DâAlessio DA, Fradkin J, Kernan WN, Mathieu C, Mingrone G, Rossing P, Tsapas A, Wexler DJ, Buse JB. (2018). Management of hyperglycemia in type 2 diabetes, 2018. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care. 41:2669-2701.
- del Olmo-Garcia MI, Merino-Torres JF. (2018). GLP 1 receptor agonists and cardiovascular disease in patients with type 2 diabetes. J Diabetes Res. 2018:4020492.
- Douros A, Filion KB, Yin H, Yu OH, Etminan M, Udell JA, Azoulay L. (2018). Glucagon-like peptide 1 receptor agonists and the risk of incident diabetic retinopathy. Diabetes Care. 41:2330-2338.
- Duan CM, Wan TF, Wang Y, Yang QW. (2019). Cardiovascular outcomes of liraglutide in patients with type 2 diabetes: A systematic review and meta-analysis. Medicine. 98:e17860.
- Elsaid MI, Li N, Firkins SA, Rustgi VK, Paskett ED, Acharya C, Reddy KR, Chiang CW, Mumtaz K. (2024). Impacts of glucagon like peptide 1 receptor agonists on the risk of adverse liver outcomes in patients with metabolic dysfunction associated steatotic liver disease cirrhosis and type 2 diabetes. Aliment Pharmacol Ther. 59:1096-1110.
- Emad M, Osama H, Rabea H, Saeed H. (2023). Dual compared with triple antithrombotics treatment effect on ischemia and bleeding in atrial fibrillation following percutaneous coronary intervention: A meta-analysis. Int J Clin Med Res. 1:77-87.
- Gaborit B, Julla JB, Besbes S, Proust M, Vincentelli C, Alos B, Ancel P, Alzaid F, Garcia R, Mailly P, Sabatier F. (2020). Glucagon-like peptide 1 receptor agonists, diabetic retinopathy and angiogenesis: The AngioSafe type 2 diabetes study. J Clin Endocrinol Metab. 105:e1549-1560.
- Gaborit B, Julla JB, Besbes S, Proust M, Vincentelli C, Alos B, Ancel P, Alzaid F, Garcia R, Mailly P, Sabatier F. (2021). Design and baseline characteristics of the AMPLITUDE O cardiovascular outcomes trial of efpeglenatide, a weekly glucagon like peptide 1 receptor agonist. Diabetes Obes Metab. 23:318-323.
- Gerstein HC, Colhoun HM, Dagenais GR, Diaz R, Lakshmanan M, Pais P, Probstfield J, Riesmeyer JS, Riddle MC, Rydén L, Xavier D. (2019). Dulaglutide and cardiovascular outcomes in type 2 diabetes (REWIND): A double-blind, randomised placebo-controlled trial. The Lancet. 394:121-130.
- Gupta A, Das A, Majumder K, Arora N, Mayo HG, Singh PP, Beg MS, Singh S. (2018). Obesity is independently associated with increased risk of hepatocellular cancer-related mortality: A systematic review and meta-analysis. Am J Clin Oncol. 41:874-881.
- Hernandez AF, Green JB, Janmohamed S, DâAgostino RB, Granger CB, Jones NP, Leiter LA, Rosenberg AE, Sigmon KN, Somerville MC, Thorpe KM. (2018). Albiglutide and cardiovascular outcomes in patients with type 2 diabetes and cardiovascular disease (Harmony Outcomes): A double-blind, randomised placebo-controlled trial. The Lancet. 392:1519-1529.
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. (2003). Measuring inconsistency in meta-analyses. BMJ. 327:557-560.
- Holman RR, Bethel MA, Mentz RJ, Thompson VP, Lokhnygina Y, Buse JB, Chan JC, Choi J, Gustavson SM, Iqbal N, Maggioni AP. (2017). Effects of once-weekly exenatide on cardiovascular outcomes in type 2 diabetes. N Engl J Med. 377:1228-1239.
- Huang WC, Chen YC, Wu CH, Ko Y. (2021). Cardiovascular outcomes and healthcare costs of liraglutide versus basal insulin for type 2 diabetes patients at high cardiovascular risk. Sci Rep. 11:1430.
- Husain M, Birkenfeld AL, Donsmark M, Dungan K, Eliaschewitz FG, Franco DR, Jeppesen OK, Lingvay I, Mosenzon O, Pedersen SD, Tack CJ. (2019). Oral semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 381:841-851.
- Jorsal A, Kistorp C, Holmager P, Tougaard RS, Nielsen R, Hänselmann A, Nilsson B, Møller JE, Hjort J, Rasmussen J, Boesgaard TW. (2017). Effect of liraglutide, a glucagon like peptide 1 analogue, on left ventricular function in stable chronic heart failure patients with and without diabetes (LIVE)-a multicentre, double blind, randomised, placebo controlled trial. Eur J Heart Fail. 19:69-77.
- Kristensen SL, Rørth R, Jhund PS, Docherty KF, Sattar N, Preiss D, Køber L, Petrie MC, McMurray JJ. (2019). Cardiovascular, mortality, and kidney out- comes with GLP-1 receptor agonists in patients with type 2 diabetes: A systematic review and meta-analysis of cardiovascular outcome trials. Lancet Diabetes Endocrinol. 7:776-785.
- Kumar R, Sharma S, Chattopadhyay S. (2018). Momordicacharantia: From traditional use to evidence-based medicine. PharmacolRes. 132:110-131.
- Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, Clarke M, Devereaux PJ, Kleijnen J, Moher D. (2009). The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: Explanation and elaboration. BMJ. 339:b2700.
- Lund A, Knop FK, Vilsbøll T. (2014). Glucagon-like peptide-1 receptor agonists for the treatment of type 2 diabetes: Differences and similarities. Eur J Intern Med. 25:407-14.
- Madsbad S. (2016). Review of head to head comparisons of glucagon like peptide 1 receptor agonists. Diabetes Obes Metab. 18:317-32.
- Margulies KB, Hernandez AF, Redfield MM, Givertz MM, Oliveira GH, Cole R, Mann DL, Whellan DJ, Kiernan MS, Felker GM, McNulty SE. (2016). Effects of liraglutide on clinical stability among patients with advanced heart failure and reduced ejection fraction: A randomized clinical trial. JAMA. 316:500-508.
- Marre MU, Shaw J, Brändle M, Bebakar WW, Kamaruddin NA, Strand J, Zdravkovic M, Le Thi TD, Colagiuri S, LEAD 1 SU study group. (2009). Liraglutide, a once daily human GLP 1 analogue, added to a sulphonylurea over 26 weeks produces greater improvements in glycaemic and weight control compared with adding rosiglitazone or placebo in subjects with type 2 diabetes (LEAD 1 SU). Diabetic Medicine. 26:268-278.
- Marso SP, Bain SC, Consoli A, Eliaschewitz FG, Jódar E, Leiter LA, Lingvay I, Rosenstock J, Seufert J, Warren ML, Woo V. (2016). Semaglutide and car- diovascular outcomes in patients with type 2 diabetes. N Engl J Med. 375:1834-1844.
- Marso SP, Daniels GH, Brown-Frandsen K, Kristensen P, Mann JF, Nauck MA, Nissen SE, Pocock S, Poulter NR, Ravn LS, Steinberg WM. (2016). Liraglu- tide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 375:311-322.
- Marsico F, Paolillo S, Gargiulo P, Bruzzese D, DellâAversana S, Esposito I, Renga F, Esposito L, Marciano C, Dellegrottaglie S, Iesu I. (2020). Effects of glucagon-like peptide-1 receptor agonists on major cardiovascular events in patients with Type 2 diabetes mellitus with or without established cardio-vascular disease: A meta-analysis of randomized controlled trials. Eur Heart J. 41:3346-58.
- Men P, Qu S, Song Z, Liu Y, Li C, Zhai S. (2020). Lixisenatide for type 2 diabetes mellitus patients inadequately controlled on oral antidiabetic drugs: A mixed-treatment comparison meta-analysis and cost-utility analysis. Diabetes Therapy. 11:1745-1755.
- Pfeffer MA, Claggett B, Diaz R, Dickstein K, Gerstein HC, Køber LV, Lawson FC, Ping L, Wei X, Lewis EF, Maggioni AP. (2015). Lixisenatide in patients with type 2 diabetes and acute coronary syndrome. N Engl J Med. 373:2247-2257.
- Rao PV, Gan SH. (2014). Cinnamon: A multifaceted medicinal plant. Evid Based Complement Alternat Med. 2014:642942.
- Read PA, Khan FZ, Dutka DP. (2012). Cardioprotection against ischaemia induced by dobutamine stress using glucagon-like peptide-1 in patients with coronary artery disease. Heart. 98:408-13.
- Potential role
- Sayed AN, Abdallah RA, Abdallah RA, Sayed AN, Fares MY. (2023). Compliance, public knowledge, perceptions, and attitude of Egyptians people towards COVID-19: A cross-sectional study. Int J Clin Med Res. 1:123-131.
- Singh J, Kakkar P. (2014). Modulation of diabetes mellitus by natural products through regulation of nuclear receptors. Nutr Metab. 11:1-14.
- Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, Moher D, Becker BJ, Sipe TA, Thacker SB. (2000). Meta-analysis of observational studies in epidemiology: A proposal for reporting. JAMA. 283:2008-12.
- Tiwari P, Mishra BN, Sangwan NS. (2014). Phytochemical and pharmacological properties of Gymnema sylvestre: An important medicinal plant. Biomed Res Int. 2014:830285.
- Yen FS, Hou MC, Wei JC, Shih YH, Hwu CM, Hsu CC. (2024). Effects of glucagon-like peptide-1 receptor agonists on liver-related and cardiovascular mortality in patients with type 2 diabetes. BMC Med. 22:8.
- Zelniker TA, Wiviott SD, Raz I, Im K, Goodrich EL, Furtado RH, Bonaca MP, Mosenzon O, Kato ET, Cahn A, Bhatt DL. (2019). Comparison of the effects of glucagon-like peptide receptor agonists and sodium-glucose cotransporter 2 inhibitors for prevention of major adverse cardiovascular and renal outcomes in type 2 diabetes mellitus: Systematic review and meta-analysis of cardiovascular outcomes trials. Circulation. 139:2022-2031.
- Zelniker TA, Wiviott SD, Raz I, Im K, Goodrich EL, Bonaca MP, Mosenzon O, Kato ET, Cahn A, Furtado RH, Bhatt DL. (2019). SGLT2 inhibitors for primary and secondary prevention of cardiovascular and renal outcomes in type 2 diabetes: A systematic review and meta-analysis of cardiovascular outcome trials. The Lancet. 393:31-39.