Research - Modern Phytomorphology ( 2025) Volume 19, Issue 3
Assessment of Cry1Ac and Cry2A expression endotoxins in transgenic cotton across growth periods and stages under semi-controlled conditions
Mahreen Hanif1, Shafqat Saeed1*, Zuluqrnain Khan2, Mudssar Ali1 and Muhammad Ishtiaq12Institute of Plant Breeding and Biotechnology, MNS University of Agriculture, Multan, Pakistan
Shafqat Saeed, Institute of Plant Protection, MNS University of Agriculture, Multan, Pakistan, Email: shafqat.saeed@mnsuam.edu.pk, mahreenhanif2@gmail.com
Received: 01-Mar-2025, Manuscript No. mp-25-162560; Accepted: 26-Mar-2025, Pre QC No. mp-25-162560 (PQ); Editor assigned: 05-Mar-2025, Pre QC No. mp-25-162560 (PQ); Reviewed: 15-Mar-2025, QC No. mp-25-162560 (Q); Revised: 21-Mar-2025, Manuscript No. mp-25-162560 (R); Published: 05-Apr-2025, DOI: 10.5281/zenodo.15093541
Abstract
Transgenic cotton has brought about a significant transformation in the management of bollworms. Nevertheless, a considerable challenge has emerged in the form of Pectinophora gossypiellafor transgenic cotton. In current study, the detection and quantification of Cry1Ac and Cry2Ab protein in pot samples of different transgenic cotton cultivars encompassing single (Cry1Ac) and double gene (Cry2Ab) cultivars was conducted, employing bt strips and ELISA methods at three distinct time points, namely 40 days, 80 days, and 120 days after sowing, coinciding with different growth phases (i.e., leaves, squares, bolls, and seeds). The outcomes revealed that the highest toxin levels of Cry1Ac protein were present across all cultivars, whereas elevated levels of Cry2Ab protein were exclusively observed in MNH-1045 cultivar ranged from 0.05 ug/g to 1.46 ug/g. The findings highlighted visible differences in the concentrations of Cry1Ac and Cry2Ab proteins among all cultivars. The Cry1Ac concentrations ranged from 0.12 ug/g to 0.81 ug/g, 0.18 ug/g to 0.69 ug/g, 0.19 ug/g to 0.38 ug/g after 40 days, 80 days, and 120 days respectively, and ranged from 0.22 ug/g to 0.59 ug/g, 0.20 ug/g to 0.36 ug/g, 0.00 ug/g to 0.05 ug/g and 0.22 ug/g to 0.30 ug/g, in leaf, square, boll, and seed stage respectively. The concentration of Cry2Ab protein ranged from 0.04 to 1.46, 0.02 to 1.128, 0.01 to 0.28ug/g after 40 days, 80 days, and 120 days respectively, and ranged from 0.4 ug/g to 1.45 ug/g, 0.01 ug/g to 0.63 ug/g, 0.00 ug/g to 0.05 ug/g and 0.00 ug/g to 0.62 ug/g, in leaf, square, boll, and seed stage respectively. It was detected that the expression of toxins is depending upon the developmental stage of the crop and the duration of growth. This study's findings will aid entomologists and plant breeders in developing high-toxin cotton cultivars (especially expressing high at the boll stage) and strategies for global cotton production sustainability, such as refuge maintenance or hybrid development, to protect transgenic cotton from pink bollworm infestations.
Keywords
Bt cotton, Bt toxin, Cry1Ac detection, Cry2Ab detection, Growth periods, Growth stages, Quantification
Introduction
Globally, farmers allocate approximately 80% of the total pesticide usage towards the control of bollworms in cotton crops (Arshad & Suhail 2011). The extensive reliance on pesticides to combat cotton insect pests, particularly Helicoverpa armigera, has led to diminishing pesticide efficacy, reduced farmer income, escalated cotton production costs, and the emergence of resistance issues (Forrester et al., 1993; Yang et al., 2000; Lu et al., 2012). Researchers have identified an effective solution for mitigating insecticide resistance in these pests, which involves the utilization of biological insecticides containing Bacillus thuringiensis (Schnepf et al., 1998). Subsequently, transgenic Bt cotton was developed, incorporating Bacillus thuringiensis endotoxins, specifically the Cry1Ac toxin, which has demonstrated remarkable effectiveness in managing cotton bollworms and other lepidopteran pests (Wilson et al., 1992; Flint et al., 1995; Hutchison 1999; Mendelsohn et al., 2003; Wu & Guo 2005). The commercialization of transgenic Bt cotton commenced in the USA in 1996 and spread to other nations, including China, Australia, Mexico, India, Argentina, South Africa, Colombia, and Brazil (James 2006). In Pakistan, it gained widespread adoption by 2010. Transgenic cotton offers multiple advantages to farmers, including reductions in pesticide usage, lower pest incidence, enhanced cotton sustainability, reduced production expenses, preservation of beneficial fauna, and increased yields (Godfray et al., 2010; Abedullah et al., 2015; Naseem and Qaim 2016). In China, the adoption of Bt cotton has resulted in a significant reduction in insecticide applications, ranging from 47% to 79% (Veettil et al., 2017).
Pectinophora gossypiellaexhibits a heightened prevalence on transgenic cotton, primarily attributable to its monophagous feeding habits (Pogue 2004). The larvae of this pest primarily consume cotton flowers and bolls (Mapuranga et al. 2015). However, owing to the widespread cultivation of Bt cotton and the limited feeding on cotton by P. gossypiella, field populations of this species have developed resistance against transgenic cotton. Resistance evolution in pink bollworms has been documented in countries such as the USA, China, and India (Carrière et al. 2010; Tabashnik et al. 2013, 2019; Jin et al. 2015; Sansinenea 2019). In laboratory settings, researchers have identified four recessive mutant alleles of cadherin responsible for conferring resistance against Bt toxins, particularly Cry1Ac (Morin et al. 2003; Fabrick & Tabashnik 2012; Fabrick et al. 2014). Pakistan and other developing nations face an elevated risk due to the extensive cultivation of unapproved Bt cotton genotypes without adherence to proper refuge recommendations. In Pakistan, an outbreak of pink bollworm infestation on Bt cultivars was reported in Vehari (Abbas et al., 2016), although there have been no published reports of such infestations in Multan.
Several factors contribute to insect resistance against transgenic cotton, including the improper placement or implementation of refuge areas and the expression levels of Bt toxins. Initially, insect resistance to Bt cotton arose due to the inadequate availability of refuge plants (Huang et al., 2011). Furthermore, the potency and efficacy of Bt toxins are contingent upon the cultivars' capacity to produce Bt proteins during each growth stage and in various plant parts or tissues (Olsen et al., 2005; Wan et al., 2012; Zaman et al., 2015; Cheema et al., 2016; Khan et al., 2018). Notably, Bt toxin concentrations diminish with plant age or maturity (Fitt 1998; Holt 1998; Sachs et al., 1998; Greenplate et al., 2000; Adamczyk et al., 2001; Zaman et al., 2015). Research findings have consistently indicated that leaves exhibit higher levels of toxin expression compared to fruiting parts (Greenplate et al. 2000; Adamczyk et al., 2001; Abel et al., 2004; Bakhsh et al., 2010, 2012). This reduction in toxin expression correlates with the age of the transgenic crops (Brévault et al., 2012).
The management of insect resistance to Bt cotton can be effectively achieved through the cultivation of double-gene Bt cotton (incorporating Cry1Ac and Cry2Ab) or triple-gene Bt cotton (containing Cry1Ac, Cry2Ab, and Vip3Aa). This strategy is recognized as a vital approach for delaying the development of resistance to Bt (Kranthi et al., 2005). Some research studies have reported that the evolution of resistance can be significantly delayed through the use of refuges (Carrière and Tabashnik 2001; Tabashnik et al., 2003; Yang et al., 2014). The current investigation aims to assess the influence of various growth periods (specifically, 40 days, 80 days, and 120 days after sowing) and different plant parts (including leaves, square bolls, and seed cotton) on the expression of Cry1Ac and Cry2A protein within various single, double, and triple gene cotton cultivars. In contemporary agricultural scenarios, the outcomes of such research endeavors hold significant importance. These findings are expected to contribute valuable insights for the development of more effective strategies aimed at managing pink bollworm infestations during the cropping season.
Materials and Methods
Cotton cultivars
Various transgenic cotton cultivars were sourced from distinguished cotton research institutions, which encompassed the Cotton Research Station (CRS), Central Cotton Research Institute (CCRI), and Pakistan Central Cotton Committee (PCCC). These cotton cultivars comprised both single and double gene Bt cotton cultivars, as detailed in tab. 1. Subsequently, these selected cultivars were cultivated in pots under shade (semi-controlled conditions) within the premises of the University at MNS University of Agriculture Multan. From this initial selection, six cultivars were chosen to proceed with further trials, distinguished by their notably high Cry1Ac protein concentrations in leaf samples.
| Cultivar1 | Source3 | |
|---|---|---|
| Cry1Ac | NS-211 | PCCC |
| CIM-598 | CCRI | |
| Cry1Ac + Cry2Ab | Weal-AG-201 | PCCC |
| Badar-3 | PCCC | |
| CEMB Klean Cotton-6 | PCCC | |
| MNH-1045 | CRS |
Note: 1. Cotton cultivars planted in pots include NS-211 (Cry1Ac Bt cotton), CIM-598 (Cry1Ac Bt cotton), Weal AG-201 (Cry1Ac+Cry2Ab Bt cotton), Badar-3 (Cry1Ac+Cry2Ab Bt cotton), CEMB Klean Cotton-6 (Cry1Ac+Cry2Ab Bt cotton), MNH-1045 (Cry1Ac+Cry2Ab Bt cotton).
2. C5 and C6 were transgenic “triple gene” cotton produces Cry1Ac and Cry2Ab Bt proteins as well as 5-enolpyruvylshikimate-3-phosphate synthase (EPSPS), which is responsible for glyphosate herbicide tolerance. 3. PCCC stands for Pakistan Central Cotton Committee, CRS stands for Cotton Research Station; CCRI stands for Central Cotton Research Institute.
Table 1. List of transgenic Bt cotton cultivars grown in pots.
Cry1Ac and Cry2Ab test by Bt Strips
Bt testing was conducted utilizing double-gene (Envirologix USA Quickstix Combo Strips for Cry1Ac & Cry2Ab) and triple-gene Bt strips (Envirologix USA Quickstix Combo Strips for Cry1Ac, Cry2Ab & CP4 EPSPS), following the prescribed procedure accompanying the strips to confirm the presence of Cry1Ac, Cry2Ab, and GTG genes. Leaf samples were collected from the 3rd node (Olsen et al. 2005; Yuan et al. 2012) at intervals of 40 days, 80 days, and 120 days from potted plants. Similarly, samples of leaves, squares, bolls, and seeds from potted plants were collected at various stages of plant growth. Following collection, all samples (including leaves, squares, bolls, and seeds) were promptly stored in a freezer set at -80°C.
For Bt testing, samples were carefully taken and manually ground in an extraction buffer composed of distilled water and the buffer supplied with the Bt strips, mixed in a ratio of 9:1. Subsequently, the ground samples were placed in Eppendorf tubes and subjected to Bt strip testing. After 30 seconds, the appearance or absence of a band on the strip indicated the presence or absence of Bt toxins, respectively.
Quantification of Cry1Ac and Cry2Ab by ELISA Kit
The collected samples, including leaves, squares, bolls, and seeds, were collected, leaf samples were collected from the 3rd node (Olsen et al., 2005; Yuan et al., 2012) at 40 days, 80 days, and 120 Days After Sowing (DAS). After collection, the samples were promptly kept at -80°C. To quantify the Cry1Ac and Cry2Ab proteins, Envirologix QuantiPlate ELISA kits for Cry1Ac and Cry2Ab (Envirologix, Portland, ME, USA) were used, by following the procedure described by Dohare and Tank, (2014). All necessary materials were supplied within the ELISA kit, except the wash and extraction buffers. The extraction and wash buffers were prepared by the guidelines provided in the ELISA kit instructions, specifically from the Envirologix Cry1Ac kit and Cry2Ab kit. The ELISA kits were employed following the methodology outlined by Dohare and Tank (Dohare and Tank 2014; Hanif et al., 2024).
Samples were taken and ground using a mortar and pestle, with the resulting mixture placed into Eppendorf tubes. To measure the results, an ELISA reader was utilized to obtain readings at 450 nm wave length. Non-linear regression was used to find polynomial equations from each standard curve for Cry1Ac and Cry2Ab. The Cry toxin concentration for each test sample was determined by solving each corresponding quadratic equation after the control/blank absorbance was corrected. The standards included with each ELISA kit were used to determine standard curves for each toxin, including 0 ng/g, 4 ng/g, 8 ng/g, and 16 ng/g, also denoted as parts per billion ppb, for Cry1Ac and 0 ng/g, 1 ng/g, 5 ng/g, and 10 ng/g (or ppb) for Cry2Ab.
Statistical analysis
All the data obtained from the ELISA kit were subjected to analysis by computing their respective means. Subsequently, means were compared employing Tukey's honestly significant difference test (LSD) test, facilitated by the use of Statistics 8.1 Software.
Results
Cry1Ac and Cry2Ab detection by Bt Strips
During the immunoblot strip test conducted on leaf samples, it was observed that all cultivar leaf samples consistently yielded false positive results (which means Cry1Ac protein was detected in samples) for the Cry1Ac protein in potted plants, across various time points after sowing (specifically, at 40 days, 80 days, and 120 days). However, false negative results were obtained for Cry2Ab protein (which means Cry2Ab protein was not detected in samples), except in the case of the MNH-1045 cultivar, as detailed in tab. 2.
| Cry1Ac | Cry2Ab | GTG2 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 40 DAS | 80 DAS | 120 DAS | 40 DAS | 80 DAS | 120 DAS | 40 DAS | 80 DAS | 120 DAS | |
| NS-2111 | 3 | + | + | NT4 | NT | NT | NT | NT | NT |
| CIM-598 | + | + | + | NT | NT | NT | NT | NT | NT |
| Weal AG-201 | + | + | + | - | - | - | NT | NT | NT |
| (Badar-3 | + | + | + | - | - | - | NT | NT | NT |
| CKC-6 | + | + | + | - | - | - | + | + | + |
| MNH-1045 | + | + | + | + | + | + | + | + | 1 |
NOTE: 1. Cotton cultivars planted in pots include NS-211 (Cry1Ac Bt cotton), CIM-598 (Cry1Ac Bt cotton), Weal AG-201 (Cry1Ac+Cry2Ab Bt cotton), Badar-3 (Cry1Ac+Cry2Ab Bt cotton), CEMB Klean Cotton-6 (Cry1Ac+Cry2Ab Bt cotton), MNH-1045 (Cry1Ac+Cry2Ab Bt cotton).
2. GTG stands for glyphosate tolerance gene.
3. +ve or -ve stands for presence or absence of respected toxin.
4. NT stands for not tested because the cultivar does not contain it.
Table 2. Immunoblot strip test of cultivars after different days of sowing.
The analysis of Cry1Ac and Cry2Ab detection at various growth stages, including leaf, square, bolls, and seed cotton, consistently revealed false positive results for Cry1Ac protein across all cultivars, as presented in tab. 3. All tested cultivars show positive results for Cry1Ac at leaf, square, boll and seed stages. While for Cry2Ab, CKC-6 and MNH-1045 show positive results at leaf, and boll stages than other cultivars. However, at square stage, only MNH10-45 has more positive results than other treatments. As the crop advanced to the boll stage, cultivars displayed mixed responses, with some registering positive results for Cry2Ab and others negative results among tested cultivars. Finally, at the seed stage, all cultivars consistently demonstrated a positive response. Notably, all tested cultivar displayed positive results for GTG protein at leaf, square, boll, and seed stages.
| Cry1Ac | Cry2Ab | GTG2 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Leaf | Square | Boll | Seed | Leaf | Square | Boll | Seed | Leaf | Square | Boll | Seed | |
| NS-2111 | +3 | + | + | + | NT4 | NT | NT | NT | NT | NT | NT | NT |
| CIM-598 | + | + | + | + | NT | NT | NT | NT | NT | NT | NT | NT |
| Weal AG-201 | + | + | + | + | - | - | + | + | NT | NT | NT | NT |
| (Badar-3 | + | + | + | + | - | - | - | + | NT | NT | NT | NT |
| CKC-6 | + | + | + | + | + | - | + | + | + | + | + | + |
| MNH-1045 | + | + | + | + | + | + | + | + | + | + | + | 1 |
NOTE: 1. Cotton cultivars planted in pots include NS-211 (Cry1Ac Bt cotton), CIM-598 (Cry1Ac Bt cotton), Weal AG-201 (Cry1Ac+Cry2Ab Bt cotton), Badar-3 (Cry1Ac+Cry2Ab Bt cotton), CEMB Klean Cotton-6 (Cry1Ac+Cry2Ab Bt cotton), MNH-1045 (Cry1Ac+Cry2Ab Bt cotton).
2. GTG stands for glyphosate tolerance gene.
3. +ve or -ve stands for presence or absence of respected toxin.
4. NT stands for not tested because the cultivar does not contain it.
Table 3. Immunoblot strip test of cultivars at different crop growth stages.
Quantification of Cry1Ac and Cry2Ab by ELISA kit
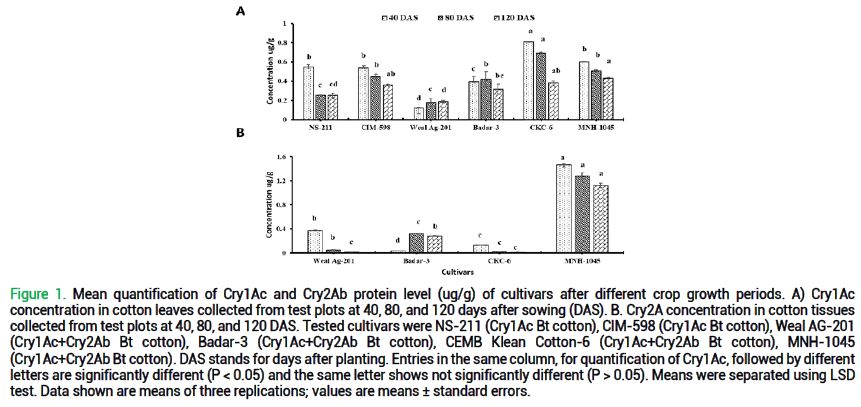
Quantification of Cry1Ac and Cry2Ab protein after different days of sowing: Cry1Ac quantification was conducted on leaf samples obtained from both field-grown and potted plants of all cultivars at various time points following sowing/planting, specifically at 40 days, 80 days, and 120 days, as indicated in fig. 1. A highly significant difference was detected in the levels of Cry1Ac protein among leaf samples of all cultivars at 40 days after sowing (F=56.4; df=5, 12; p<0.000) and 80 days after sowing (F=22.4; df=5, 12; p<0.000) under field conditions. However, no significant difference was observed at 120 days after sowing (F=9.05; df=5, 12; p<0.000). At the 40-day, leaf samples of CKC-6 cultivar exhibited the highest concentration of Cry1Ac protein compared to all other cultivars. The concentration of Cry1Ac protein (measured in micrograms per gram, ug/g) ranged from 0.12 ug/g to 0.81 ug/g, 0.18 ug/g to 0.69 ug/g, and 0.19 ug/g to 0.38 ug/g after 40 days, 80 days, and 120 days respectively. In some cultivars, concentration was decreased with respect to increasing days after sowing as NS-211, CIM-598, CKC-6, Badar-3, and MNH-1045. Conversely, Weal Ag-201 demonstrated elevated levels of Cry1Ac protein after 80 days and 120 days. However, Badar 3 demonstrated elevated levels of Cry1Ac protein after 80 and then decreased after 120 days.
Figure 1. Mean quantification of Cry1Ac and Cry2Ab protein level (ug/g) of cultivars after different crop growth periods. A) Cry1Ac concentration in cotton leaves collected from test plots at 40, 80, and 120 days after sowing (DAS). B. Cry2A concentration in cotton tissues collected from test plots at 40, 80, and 120 DAS. Tested cultivars were NS-211 (Cry1Ac Bt cotton), CIM-598 (Cry1Ac Bt cotton), Weal AG-201 (Cry1Ac+Cry2Ab Bt cotton), Badar-3 (Cry1Ac+Cry2Ab Bt cotton), CEMB Klean Cotton-6 (Cry1Ac+Cry2Ab Bt cotton), MNH-1045 (Cry1Ac+Cry2Ab Bt cotton). DAS stands for days after planting. Entries in the same column, for quantification of Cry1Ac, followed by different letters are significantly different (P < 0.05) and the same letter shows not significantly different (P > 0.05). Means were separated using LSD test. Data shown are means of three replications; values are means ± standard errors.
The quantification of Cry2Ab protein levels in all cultivars was conducted at various time points following sowing/planting, specifically at 40 days, 80 days, and 120 days. The samples were collected from potted plants and are presented. Notably, an extremely significant difference was observed in the Cry2Ab protein levels within leaf samples of all cultivars at 40 days after sowing (F=1500; df=3, 8; p<0.000), 80 days after sowing (F=497; df=3, 8; p<0.000) and at 120 days after sowing (F=448; df=3, 8; p<0.000) for field-grown plants. Among all the cultivars, the MNH-1045 cultivar exhibited the highest protein levels of Cry2Ab than other cultivars after 40 days, 80 days, and 120 days. Furthermore, the concentration of Cry2Ab decreased with the passage of time in potted leaf samples of Weal Ag-201, CKC-6, and MNH-1045. However, Badar 3 demonstrated elevated levels of Cry1Ac protein after 80 days and then decreased after 120 days. The concentration of Cry2Ab protein (measured in micrograms per gram, ug/g) was ranging from 0.04 ug/g to 1.46 ug/g, 0.02 ug/g to 1.128 ug/g, and 0.01 ug/g to 0.28 ug/g after 40 days, 80 days, and 120 days respectively.
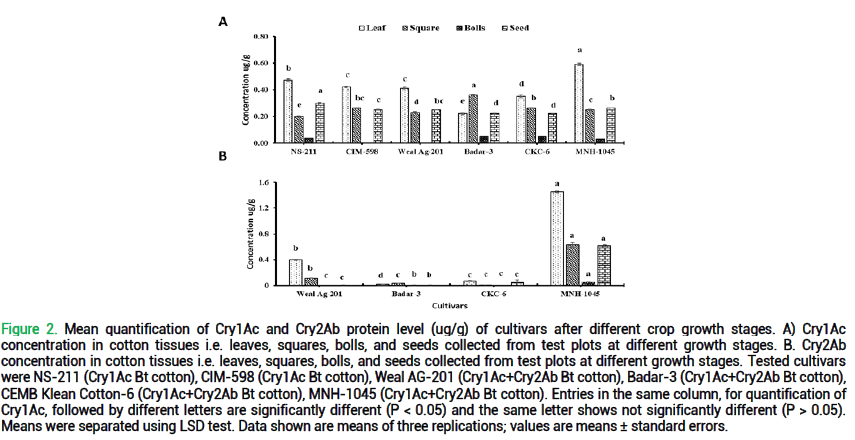
Quantification of Cry1Ac and Cry2Ab proteins at different growth stages: The quantification of Cry1Ac toxin levels was conducted across all cultivars at various growth stages, including leaf, square, boll, and seed, as outlined in fig. 2.A highly significant difference was observed in the levels of Cry1Ac protein among all cultivars at all stages, namely leaf(F=133; df=5, 12; p<0.000), square (F=140; df=5, 12; p<0.000), boll (F=M; df=5, 12; p=M), and seed (F=83.40; df=5, 12; p<0.000) under potted conditions. At the leaf stage, MNH-1045 displayed high levels of protein, while at the square stage, Badar-3 exhibited highest protein levels. Notably, at the bolls stage, lowest expression of Cry1Ac was observed among all tested cultivars. In the case of the seed stage, NS-211 demonstrated higher protein expression. Different concentrations of Cry1Ac protein were observed in samples obtained from potted plants, with levels ranging from 0.22 ug/g to 0.59 ug/g, 0.20 ug/g to 0.36 ug/g, 0.00 ug/g to 0.05 ug/g, and 0.22 ug/g to 0.30 ug/g, in leaf, square, boll, and seed stage respectively.
Figure 2. Mean quantification of Cry1Ac and Cry2Ab protein level (ug/g) of cultivars after different crop growth stages. A) Cry1Ac concentration in cotton tissues i.e. leaves, squares, bolls, and seeds collected from test plots at different growth stages. B. Cry2Ab concentration in cotton tissues i.e. leaves, squares, bolls, and seeds collected from test plots at different growth stages. Tested cultivars were NS-211 (Cry1Ac Bt cotton), CIM-598 (Cry1Ac Bt cotton), Weal AG-201 (Cry1Ac+Cry2Ab Bt cotton), Badar-3 (Cry1Ac+Cry2Ab Bt cotton), CEMB Klean Cotton-6 (Cry1Ac+Cry2Ab Bt cotton), MNH-1045 (Cry1Ac+Cry2Ab Bt cotton). Entries in the same column, for quantification of Cry1Ac, followed by different letters are significantly different (P < 0.05) and the same letter shows not significantly different (P > 0.05). Means were separated using LSD test. Data shown are means of three replications; values are means ± standard errors.
The quantification of Cry2Ab protein levels was determined across different cultivars at various growth stages, including leaf, square, boll, and seed. A highly significant difference was observed in the levels of Cry2Ab protein among all cultivars at all stages under field conditions, namely leaf (F=15959.8; df=3, 6; p<0.000), square (F=9665.77; df=3, 6; p<0.000), bolls (F=57.26; df=3, 6; p<0.000), and seed (F=37.68; df=3, 6; p=0.000). Among samples, only Weal Ag -201 and MNH-1045 exhibited the expression of Cry2Ab. Notably, the lowest level of concentrations of Cry2Ab protein was observed in other samples of tested cultivars, except MNH-1045 cultivar, with levels ranging from 0.05 ug/g to 1.45 ug/g. The MNH-1045 cultivar displayed high levels of Cry2Ab protein at the leaf stage, followed by the square, seed, and bolls stages. Conversely, in Weal Ag-201, the production of Cry2Ab protein decreased in plant growth stages from leaf to seed. The concentrations of Cry2Ab protein were ranging from 0.4 ug/g to 1.45 ug/g, 0.01 ug/g to 0.63 ug/g, 0.00 ug/g to 0.05 ug/g, and 0.00 ug/g to 0.62 ug/g, in leaf, square, boll, and seed stages respectively.
Discussion
Bollworm management initially relied on the cultivation of transgenic cotton without the use of chemical pesticides. However, due to the widespread cultivation of Bt cotton without the implementation of proper refuge strategies, bollworms developed resistance against Bt cotton (Gould 1998; Banerjee et al., 2017; Tabashnik & Carrière 2017). Another contributing factor to bollworm resistance is the variability in the expression of Cry1Ac (Jamil et al., 2021). The process of testing transgenic cotton involves cost-effective qualitative and quantitative techniques. In the current study, the qualitative detection of leaf samples from all cultivars consistently yielded positive results for the Cry1Ac protein, with the exception of Cry2Ab protein, which was detected only in the MNH10-45 and CKC-6 cultivar during various stages of crop growth (i.e., 40 days, 80 days, and 120 days). When testing different growth stages of all cultivars, the presence of Cry1Ac was observed in all samples of potted plants. However, the presence of Cry2Ab protein was only detected at the leaf, boll, and seed stages.
Quantitative analysis conducted through ELISA revealed variations in the concentration of the Cry1Ac protein among different cultivars during the crop growth period, specifically at 40 days, 80 days, and 120 days in potted plant leaf samples. Some cultivars exhibited higher Cry1Ac concentrations at 40 DAS, while others showed elevated concentrations at 80 DAS and 120 DAS. In contrast, the Cry2Ab protein concentration was highest at 40 DAS in the MNH-1045 cultivar but decreased as the crop growth period extended. Notably, in some cultivars, the concentration of the Cry2Ab protein was very low, even reaching zero. These findings align with previous research, which reported that the expression of the Cry1Ac protein in transgenic cotton decreases as the plant matures, impacting the efficacy of transgenic cotton against target insects later in the season (Zhang et al. 2001; Abel et al. 2004; Hanif et al., 2025). Indeed, there is evidence to suggest that the quantity of insecticidal proteins in Bt cotton can vary depending on factors such as the plant's age, structure, and susceptibility to environmental challenges (Dong and Li 2007).
The quantitative analysis of Cry1Ac protein concentration revealed some noteworthy patterns among different cotton cultivars at various growth stages. Specifically, at the leaf and seed stages, samples of the MNH-1045 cultivar exhibited higher concentrations of Cry1Ac protein compared to other cultivars. The Badar-3 cultivar showed a higher concentration of Cry1Ac protein at the square stage than cultivars. While all cultivars had very low concentrations of Cry1Ac protein at the bolls stage. However, all cultivars have high concentrations at seed stage. Instead of Cry1Ac concentration, Cry2Ab concentration was highest in the MNH1045 cultivar at leaf, square, boll, and seed stages. Across different growth stages, Cry1Ac proteins were documented as being more abundant in leaves during the vegetative stage compared to the reproductive stage. These findings highlight the dynamic nature of protein expression in different cotton cultivars and at different stages of plant growth, which can have implications for pest management and the effectiveness of Bt cotton in controlling insect pests (Olsen et al., 2005). Another study demonstrated that the expression of the Cry1Ac toxin was higher in the leaves than at other stages, such as squares, flowers, and bolls (Tabashnik et al., 2003; Udikeri 2006; Likhitha et al., 2023). Drought and elevated temperatures significantly diminished the quantity of insecticidal protein in bolls (Zhang et al., 2021). The expression of the toxin decreased as the plant aged and as the crop seasons progressed in transgenic crops (Kranthi et al., 2005; Carrière et al., 2019). Hence, the decline in Bt toxin expression in Bt cotton with plant maturity resulted in increased survival of the target pests (Chen et al., 2000). Bt gene expression levels exhibit variability due to factors including the location of insertion, promoter sequences, environmental conditions, genetic bases, and genetic backgrounds (Guo et al., 2001; Adamczyk & Meredith 2004; Huang et al., 2011). Abiotic environmental conditions, such as soil salinity, elevated temperatures, waterlogging, nitrogen deficiency, and humidity, have an impact on the expression of the Bt gene (Chen et al., 2021; Rahman et al., 2022; Jehangir & Ali 2023).
Pink bollworm infestation in transgenic cotton results from decreasing toxin levels over time or the evolution of resistance in pink bollworms. Field-evolved resistance to transgenic cotton with Cry1Ac was reported in pink bollworms in India (Dhurua & Gujar 2011; Fabrick et al., 2014). Likewise, reports of pink bollworm infestations in double-gene Bt cotton (containing Cry1Ac and Cry1Ab) have emerged from India (Kranthi 2015; Mohan et al., 2016; Naik et al., 2018, 2020, 2021; Annepu et al., 2023). In Pakistan, pink bollworm infestation to green boll, locule, and open bolls of single, double, and triple gene cultivars was found to be highest (Hanif et al., 2025). Resistance evaluations were conducted against various endotoxins in seven major insect pest species on a global scale (Grimi et al., 2015). Other research results indicate that infestation in fruiting bodies was more pronounced in the BollGard-I genotype than in the BollGard-II genotypes (Gore et al., 2001; Jackson et al., 2003; Bheemanna et al., 2008; Onkaramurthy et al., 2016). Refuge planting has been recognized as the most effective strategy to delay pink bollworm resistance to transgenic cotton in the United States (Tabashnik 1994; Liu and Tabashnik 1997; Tabashnik et al., 2004, 2013). This strategy was also found effective in Pakistan (Hanif et al., 2025). This approach reduces selection pressure on target insect pests like pink bollworms and extends the lifespan of Bt cotton. It is recommended to plant refuge crops (non-Bt cotton) on a large scale alongside Bt cotton to manage pink bollworm incidence (Zaman et al., 2015).
Resistance monitoring in pink bollworms began in Arizona in 1996 (Tabashnik et al., 2000). In Pakistan, while outbreaks of pink bollworm damage on Bt cotton have occurred, resistance has not been confirmed through published articles. This study has identified cultivars with insufficient toxin levels to effectively control the pest. To address this issue, it is essential to increase the concentration of Cry1Ac and Cry2A genes in transgenic cotton by following the refuge strategy and all IPM (Integrated pest management) approaches (Hanif et al., 2025; 2025). Furthermore, the development of appropriate policies and strategies, such as implementing non-Bt cotton as a refuge with matching growth stages from flowering to fruiting, is necessary for environmentally friendly management of pink bollworm resistance to transgenic cotton.
Conclusion
The study aimed to assess Cry1Ac and Cry2Ab protein levels in transgenic cotton cultivars at various plant growth stages and periods. Overall, MNH1045 has higher concentrations of Cry1Ac than Cy2Ab among tested cultivars. It also concluded that the concentration of Cry1Ac protein in the leaf remained almost consistent across all growth periods and stages but exhibited a decrease during the square and boll stages in all tested cultivars. While Cry2A levels declined over the developmental period and stages. While MNH-1045 had the highest concentration of Cry2Ab. However, protein levels depended on cultivars, growth periods (40 days, 80 days, and 120 days), and growth stages (leaf, square, bolls, and seed). Further research is needed to enhance toxin concentrations in transgenic cotton, especially at boll stage for better pest management, especially against pink bollworms in Pakistan.
Acknowledge
We thank the institute of Cotton Research Station (CRS), Central Cotton Committee (PCCC), and Mahmood Z, Central Cotton Research Institute (CCRI) Multan, for providing seed cotton.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Conflicts of Interest
There is no conflict of interest among authors. This manuscript does not contain any studies involving human participants and/or animals. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
Abbas G, Farhan M, Haq I, Ghouse G. (2016). Accelerating infestation of pink bollworm Pectinophora gossypiella (Saunders) (Lepidoptera: Gelechiidae) on Bt–varieties of cotton in Pakistan. Pak Entomol. 38:109-113. [Google Scholar][Crossref]
Abedullah, Kouser S, Qaim M. (2015). Bt Cotton, Pesticide Use and Environmental Efficiency in Pakistan. J Agric Econ. 66:66-86. [Google Scholar][Crossref]
Abel CA, Adamczyk JJ. (2004). Relative concentration of Cry1A in maize leaves and cotton bolls with diverse chlorophyll content and corresponding larval development of fall armyworm (Lepidoptera: Noctuidae) and Southwestern corn borer (Lepidoptera: Crambidae) on maize whorl leaf profile. J Econ Entomol. 97:1737-1744. [Google Scholar][Crossref]
Adamczyk JJ, Adams LC, Hardee DD. (2001). Field efficacy and seasonal expression profiles for terminal leaves of single and double Bacillus thuringiensis toxin cotton genotypes. J Econ Entomol. 94:1589-1593. [Google Scholar][Crossref]
Adamczyk JJ, Meredith J. (2004). Genetic basis for viability of Cry1Ac expression among commercial transgenic Bacillus thuringiensis (Bt) cotton cultivars in the United States. J Insect Sci. 8:17-23. [Google Scholar]
Annepu AA, Naik VCB, Prasada Rao GMV. (2023). Frequency of Cry1Ac and Cry2Ab resistance alleles in pink bollworm, Pectinophora gossypiella Saunders from Andhra Pradesh, India. Phytoparasitica. 51:491-502. [Google Scholar][Crossref]
Arshad M, Suhail A. (2011). Field and laboratory performance of transgenic Bt cotton containing Cry1Ac against beet armyworm larvae (Lepidoptera: Noctuidae). Pak J Zool. 43:529-535. [Google Scholar]
Bakhsh A, Rao AQ, Shahid AA, Husnain T, Riazuddin S. (2010). Camv 35S is a developmental promoter being temporal and spatial in expression pattern of insecticidal genes (Cry1Ac & Cry2a) in cotton. Aust J Basic Appl Sci. 4:37-44. [Google Scholar]
Bakhsh A, Siddique S, Husnain T. (2012). A molecular approach to combat spatio-temporal variation in insecticidal gene (Cry1Ac) expression in cotton. Euphytica. 183:65-74. [Google Scholar][Crossref]
Banerjee R, Hasler J, Meagher R. (2017). Mechanism and DNA-based detection of field-evolved resistance to transgenic Bt corn in fall armyworm (Spodoptera frugiperda). Sci Rep. 7:10877-10879. [Google Scholar][Crossref]
Bheemanna M, Patil BV, Hanchinal SG, Hosamani AC, Bansi AB. (2008). Comparative performance and economics of bollgaurd-II Bt cotton under irrigated conditions. J Cotton Res Dev. 22:118-121. [Google Scholar]
Brévault T, Nibouche S, Achaleke J, Carrière Y. (2012). Assessing the role of non-cotton refuges in delaying Helicoverpa armigera resistance to Bt cotton in West Africa. Evol Appl. 5:53-65. [Google Scholar][Crossref]
Carrière Y, Crowder DW, Tabashnik BE. (2010). Evolutionary ecology of insect adaptation to Bt crops. Evol Appl. 3:561-573. [Google Scholar][Crossref]
Carrière Y, Degain B, Unnithan GC, Harpold VS, Li X, Tabashnik BE. (2019). Seasonal declines in Cry1Ac and Cry2Ab concentration in maturing cotton favor faster evolution of resistance to pyramided Bt cotton in Helicoverpa zea (Lepidoptera: Noctuidae). J Econ Entomol. 112:2907-2914. [Google Scholar][Crossref]
Carrière Y, Tabashnik BE. (2001). Reversing insect adaptation to transgenic insecticidal plants. Proc R Soc B. 1836-1844. [Google Scholar][Crossref]
Cheema HMN, Khan AA, Khan MI, Aslam U, Rana IA, Khan IA. (2016). Assessment of Bt cotton genotypes for the Cry1Ac transgene and its expression. J Agric Sci. 154:109-117. [Google Scholar][Crossref]
Chen Y, Liu ZY, Tambel LIM, Zhang X, Chen DH. (2021). Reduced square Bacillus thuringiensis insecticidal protein content of transgenic cotton under N deficit. J Integr Agric. 20:100-108. [Google Scholar][Crossref]
Chen S, Wu J, Zhou B, Huang J, Zhang R. (2000). On the temporal and spatial expression of Bt toxin protein in Bt transgenic cotton. Acta Gossypii Sin.;12:189-193. [Google Scholar][Crossref]
Dhurua S, Gujar GT. (2011). Field-evolved resistance to Bt toxin Cry1Ac in the pink bollworm, Pectinophora gossypiella (Saunders) (Lepidoptera: Gelechiidae), from India. Pest Manag Sci. 67:898-903. [Google Scholar][Crossref]
Dohare A, Tank SK. (2014). Identification of Cry1Ac and Cry2Ab proteins in transgenic cotton seeds available in Gujarat (India) by ELISA method. J Exp Biol Agric Sci. 2:43-48. [Google Scholar]
Dong HZ, Li WJ. (2007). Variability of endotoxin expression in Bt transgenic cotton. J Agron Crop Sci. 193:21-29. [Google Scholar][Crossref]
Fabrick JA, Ponnuraj J, Singh A. (2014). Alternative splicing and highly variable cadherin transcripts associated with field-evolved resistance of pink bollworm to Bt cotton in India. PLoS ONE. 9: 35658. [Google Scholar][Crossref]
Fabrick JA, Tabashnik BE. (2012). Similar genetic basis of resistance to Bt toxin Cry1Ac in boll-selected and diet-selected strains of pink bollworm. PLoS ONE. 7:35658. [Google Scholar][Crossref]
Fitt GP. (1998). Efficacy of Ingard cotton on-patternes and consequence. In: In The Ninth Australian Cotton Conference Proceedings. Cotton Res. Dev. Corp. 40:233-245. [Google Scholar] [Crossref]
Flint HM, Henneberry TJ, Wilson FD, Holguin E, Parks N, Buehler RE. (1995). The effects of transgenic cotton, Gossypium hirsutum L., containing Bacillus thuringiensis toxin genes for the control of the pink bollworm, Pectinophora gossypiella (Saunders) and other arthropods. Southwest Entomol. 20: 281-292. [Google Scholar]
Forrester NW, Cahill M, Bird LJ, Layland JK. (1993). Management of pyrethroid and endosulfan resistance in Helicoverpa armigera (Lepidoptera: Noctuidae) in Australia. Bull. Entomol Res: Suppl Ser Suppl. 1: 132. [Google Scholar]
Godfray HCJ, Beddington JR, Crute IR. (2010). Food security: The challenge of feeding 9 billion people. Science 327:812-818. [Google Scholar][Crossref]
Gore J, Leonard BR, Adamczyk JJ. (2001). Bollworm (Lepidoptera: Noctuidae) survival on “Bollgard” and “Bollgard II” cotton flower bud and flower components. J Econ Entomol. 94:1445-1451. [Google Scholar][Crossref]
Gould F. (1998). Sustainability of transgenic insecticidal cultivars: Integrating pest genetics and ecology. Annu Rev Entomol. 43:701-726. [Google Scholar][Crossref]
Greenplate J, Penn SR, Mullins JW, Oppenhuizen M. (2000). Seasonal CryIAc levels in DP50B: The “Bollgard® basis” for Bollgard II. In: 2000 Proc Cotton Conf. 2.1039-1040. [Google Scholar]
Grimi D., F O, S M, Head G E. (2015). Detection and characterization of Diatraea saccharalis resistant to Cry1A. In: 105 protein in a population of northeast San Luis province in Argentina. In Congr. Argent. Entomol. [Google Scholar][Crossref]
Guo WZ, Sun J, Guo YF, Zhang TZ. (2001). Investigation of different dosages of inserted Bt genes and their insect-resistance in transgenic Bt cotton. Acta Genetica Sinica 28: 668-676. [Google Scholar]
Hanif M, Saeed S, Ali M, Ishtiaq M, Khan Z, (2025). Field assessment of Cry1Ac and Cry1Ac + Cry2A Bt cotton against pink bollworm in Pakistan. [Google Scholar][Crossref]
Hanif M, Saeed S, Ali M, Ishtiaq M, Khan Z. (2025). Evaluation of non-Bt Refugia Cultivation to Manage Bt Resistance in Pectinophora gossypiella against Transgenic Cotton. Pak J Zool. [Google Scholar][Crossref]
Holt H. 1998. Season-long monitoring of transgenic cotton plants development of an assay for the quantification of International Cotton Conference, Rationales and evolutions of cotton policies in main producing countries. In: ISSCRI International Conference. Montpellier. 11-14. [Google Scholar]
Huang F, Andow DA, Buschman LL. (2011). Success of the high-dose/refuge resistance management strategy after 15 years of Bt crop use in North America. Entomol Exp Appl. 140:1-6. [Google Scholar][Crossref]
Hutchison WD. (1999). Review and analysis of damage functions and monitoring systems for pink bollworm (Lepidoptera: Gelechiidae) in southwestern United States cotton. Southwest Entomol. 24:339-362. [Google Scholar][Crossref]
Jackson RE, Bradley JR, Van Duyn JW. (2003). Field performance of transgenic cottons expressing one or two Bacillus thuringiensis endotoxins against bollworm, Helicoverpa zea (Boddie). J Cotton Sci. 7:57-64. [Google Scholar][Crossref]
James C. (2006). Global status of commercialised biotech/GM crops: 2005. Int. Pest Control. 48. [Google Scholar]
Jamil S, Shahzad R, Rahman SU. (2021). The level of Cry1Ac endotoxin and its efficacy against H. armigera in Bt cotton at large scale in Pakistan. GM Crops Food. 12: 1-17. [Google Scholar][Crossref]
Jehangir N, Ali S. (2023). The insecticidal efficacy and performance of Bt Cotton under variable abiotic stresses–A Review on recent findings. Plant Stress. 8:100151. [Google Scholar]
Jin L, Zhang H, Lu Y. (2015). Large-scale test of the natural refuge strategy for delaying insect resistance to transgenic Bt crops. Nat Biotechnol. 33:169-174. [Google Scholar][Crossref]
Khan MI, Khan AA, Cheema HMN, Khan RSA. (2018). Spatio-temporal and intra-plant expression variability of insecticidal gene (Cry1Ac) in upland cotton. Int J Agric Biol. 20:715-722. [Google Scholar][Crossref]
Kranthi KR. (2015). Pink bollworm strikes Bt-cotton. Cotton Stat News. 37:1-6. [Google Scholar]
Kranthi KR, Naidu S, Dhawad CS. (2005). Temporal and intra-plant variability of Cry1Ac expression in Bt-cotton and its influence on the survival of the cotton bollworm, Helicoverpa armigera (Hübner) (Noctuidae: Lepidoptera). Curr Sci. 89: 291-298. [Google Scholar]
Likhitha P, Undirwade DB, Kulkarni US, Kolhe AV, Moharil MP. (2023). Cry toxin expression in different plant parts of Bt cotton at different phenological stages. Egypt J Biol Pest Control. 33:1-7. [Google Scholar][Crossref]
Liu YB, Tabashnik BE. (1997). Experimental evidence that refuges delay insect adaptation to Bacillus thuringiensis. Proc R Soc B: Biol Sci. 264:605-610. [Google Scholar][Crossref]
Lu Y, Wu K, Jiang Y, Guo Y, Desneux N. (2012). Widespread adoption of Bt cotton and insecticide decrease promotes biocontrol services. Nature. 487: 362-365. [Google Scholar][Crossref]
Mapuranga R, Chapepa B, Mudada N. (2015). Strategies for integrated management of cotton bollworm complex in Zimbabwe: A review. Int J Agron Agric Res. 7:25-35. [Google Scholar]
Mendelsohn M, Kough J, Vaituzis Z, Matthews K. (2003). Are Bt crops safe? Nat Biotechnol. 21:1003-1009. [Google Scholar][Crossref]
Mohan KS, Ravi KC, Suresh PJ, Sumerford D, Head GP. (2016). Field resistance to the Bacillus thuringiensis protein Cry1Ac expressed in Bollgard® hybrid cotton in pink bollworm, Pectinophora gossypiella (Saunders), populations in India. Pest Manag Sci. 72: 738-746. [Google Scholar][Crossref]
Morin S, Biggs RW, Sisterson MS. (2003). Three cadherin alleles associated with resistance to Bacillus thuringiensis in pink bollworm. Proc Natl Acad Sci U. S. A. 100:5004-5009. [Google Scholar][Crossref]
Naik VCB, Kb S, Kranthi S. (2021). Pink bollworm, Pectinophora gossypiella (Saunders) (Lepidoptera: Gelechiidae) survival on transgenic cotton in India. Egypt J Biol Pest Control. 31:1-7. [Google Scholar][Crossref]
Naik VCB, Kumbhare S, Kranthi S, Satija U, Kranthi KR. (2018). Field-evolved resistance of pink bollworm, Pectinophora gossypiella (Saunders) (Lepidoptera: Gelechiidae), to transgenic Bacillus thuringiensis (Bt) cotton expressing crystal 1Ac (Cry1Ac) and Cry2Ab in India. Pest Manag Sci. 74:2544-2554. [Google Scholar][Crossref]
Naik VCB, Pusadkar PP, Waghmare ST. (2020). Evidence for population expansion of Cotton pink bollworm Pectinophora gossypiella (Saunders) (Lepidoptera: Gelechiidae) in India. Sci Rep. 10:4740. [Google Scholar][Crossref]
Naseem A, Qaim M. (2016). Genetically Modified Crops and Agricultural Development. Am J Agric Econ. 98. [Google Scholar]
Olsen KM, Daly JC, Holt HE, Finnegan EJ. (2005). Season-long variation in expression of Cry1Ac gene and efficacy of Bacillus thuringiensis toxin in transgenic cotton against Helicoverpa armigera (Lepidoptera: Noctuidae). J Econ Entomol. 98: 1007-1017. [Google Scholar][Crossref]
Onkaramurthy SG, Goud KB, Udikeri SS. (2016). Field performance of second generation (BG-II) Bt cotton genotypes against bollworm complex under rainfed conditions. J Phytopathol Pest Manag. 3:12-20. [Google Scholar]
Pogue MG. (2004). A new synonym of Helicoverpa zea (Boddie) and differentiation of adult males of H. zea and H. armigera (Hübner) (Lepidotera: Noctuidae: Heliothinae. Ann Entomol Soc Am. 97:1222-1226. [Google Scholar][Crossref]
Rahman, S.U., Nazir S, Habib I, Younas M and, Mahmood K. (2022). Effects of temperature stresses and growth stages on Cry1Ac protein accumulation in Bollgard TM cotton. J Agric Food. 3: .63-71. [Google Scholar][Crossref]
Sachs ES, Benedict JH, Stelly DM. (1998). Expression and segregation of genes encoding cryIA insecticidal proteins in cotton. Crop Sci. 38:1-11. [Google Scholar][Crossref]
Sansinenea E. (2019). Applications and Patents of Bacillus spp. in Agriculture. In: Intellect Prop Issues Microbiol. 133-14. [Google Scholar][Crossref]
Schnepf E, Crickmore N, Van Rie J. (1998). Bacillus thuringiensis and Its Pesticidal Crystal Proteins. Microbiol Mol Biol Rev. 62:775-806. [Google Scholar][Crossref]
Tabashnik BE. (1994). Delaying insect adaptation to transgenic plants: Seed mixtures and refugia reconsidered. Proc. R. Soc. B: Biol. Sci. 255:7-12. [Google Scholar][Crossref]
Tabashnik BE, Brévault T, Carrière Y. (2013). Insect resistance to Bt crops: Lessons from the first billion acres. Nat Biotechnol. 31:510-521. [Google Scholar][Crossref]
Tabashnik B., Carrière Y. (2017). Surge in insect resistance to transgenic crops and prospects for sustainability. Nat Biotechnol. 35:926-935. [Google Scholar][Crossref]
Tabashnik BE, Carrière Y, Dennehy TJ. (2003). Insect resistance to transgenic Bt crops: Lessons from the laboratory and field. J Econ Entomol. 96:1031-1038. [Google Scholar][Crossref]
Tabashnik BE, Carrière Y, Gassmann A. (2019). Global Patterns of Resistance to Bt Crops Highlighting Pink Bollworm in the United States, China, and India. J Econ Entomol. 112: 2513-2523. [Google Scholar][Crossref]
Tabashnik BE, Gould F, Carrière Y. 2004. Delaying evolution of insect resistance to transgenic crops by decreasing dominance and heritability. J Evol Biol. 17:904-912. [Google Scholar][Crossref]
Tabashnik B, Patin A, Dennehy T. (2000). Frequency of resistance to Bacillus thuringiensis in field populations of pink bollworm. Proc Natl Acad Sci U. S. A. 97:12980-12984. [Google Scholar][Crossref]
Udikeri SS. (2006). Evaluation of new generation Bt cotton genotypes, Sustainability of Cry protein expression, computation of ETL, Effect on aphid predators and development of IPM. [Google Scholar]
Veettil PC, Krishna V V., Qaim M. (2017). Ecosystem impacts of pesticide reductions through Bt cotton adoption. Aust J Agric Resour. Econ. 61: 115-0134. [Google Scholar][Crossref]
Wan P, Huang Y, Wu H, (2012). Increased frequency of pink bollworm resistance to Bt toxin Cry1Ac in China. PLoS ONE. 7:29975. [Google Scholar][Crossref]
Wilson DF, Flint HM, Deaton RW (1992). Resistance of Cotton Lines Containing a Bacillus thuringiensis Toxin to Pink Bollworm (Lepidoptera: Gelechiidae) and other Insects. J. Econ. Entomol. 85: 1516-1521. [Google Scholar][Crossref]
Wu KM, Guo YY. (2005). The evolution of cotton pest management practices in China. Annu. Rev. Entomol. 50: 31-52. [Google Scholar][Crossref]
Yang F, Kerns DL, Head GP (2014). A challenge for the seed mixture refuge strategy in Bt maize: Impact of cross-pollination on an ear-feeding pest, corn earworm. PLoS ONE. 9: 112962. [Google Scholar][Crossref]
Yang Y, Zhu M, Wang D. (2000). Yearly changes of the control effects of pyrethrums insecticides on Pectinophora gosspiella saunders. Acta Agr Jiangsu. 16: 92-96. [Google Scholar][Crossref]
Yuan CHEN, Wen YJ, Cothren JT. (2012). Effects of Extreme Air Temperature and Humidity on the Insecticidal Expression Level of Bt Cotton. J Integr Agric. 11: 1836-1844. [Google Scholar][Crossref]
Zaman M, Mirza MS, Irem S, Zafar Y, Mehboob-ur-Rahman. (2015). A temporal expression of Cry1Ac protein in cotton plant and its impact on soil health. Int J Agric Biol. 17. [Google Scholar][Crossref]
Zhang X, Tian Q, Zhao Z, Dong Z, Chen Y, Chen D. (2021). Analysis of differentially expressed proteins affecting insecticidal protein content in Bt cotton under highâ?temperature and water deficit stress using labelâ?free quantitation. J Agron Crop Sci. 207:1-11. [Google Scholar][Crossref]
Zhang Y, Wu K, Guo Y. (2001). On the spatio-temporal expression of the contents of Bt insecticidal protein and the resistance of Bt transgenic cotton to cotton bollworm. Acta Phytophyl. 28:701-726. [Google Scholar]