Research Article - Modern Phytomorphology ( 2025) Volume 19, Issue 4
The cytotoxic effect of dwarf stinging nettle (Urtica urens) water extract on human breast cancer cells (MCF-7)
Sawsan A. Rahimuddin* and Hala S. SonbolSawsan A. Rahimuddin, Department of Biochemistry, King Abdulaziz University, Jeddah, Kingdom of Saudi Arabia, Email: zawsan@hotmail.com
Received: 14-May-2025, Manuscript No. mp-25-165499; , Pre QC No. mp-25-165499 (PQ); Editor assigned: 19-May-2025, Pre QC No. mp-25-165499 (PQ); Reviewed: 02-Jun-2025, QC No. mp-25-165499; Revised: 15-Jun-2025, Manuscript No. mp-25-165499 (R); Published: 14-Jul-2025, DOI: 10.5281/zenodo.17346030
Abstract
Urtica urens or dwarf stinging nettle, is an anticancer herb in many countries. The herb's effect on cell viability, the cytoplasmic and mitochondrial Reactive Oxygen Species (ROS) levels and its effect on the cell cycle in human breast adenocarcinoma cells (MCF-7) were investigated. The malignant cells were incubated with 2 mg/ml extracts of both freeze- dried and freshly dried leaves. After treatment, the antiproliferation changes of cells were measured using Trypan blue and MTT assays. The cytoplasmic and mitochondrial ROS were determined using flow cytometry. Finally, the type of cell death and cell cycle changes were determined using Annexin V and Propidium Iodide (PI). Results showed that both extracts reduced cell viability with reduced cytoplasmic ROS. In addition, an increase in mitochondrial ROS was observed with the freeze-dried extract. Cell cycle analysis revealed that the freshly dried leaves extract increased the early apoptosis population and arrested the cell cycle in S phase. In contrast, a significant increase in late apoptosis and necrosis phase, with cell cycle arrest in G2/M phase, was obtained with the freeze-dried leaves. Findings support that using Urtica urens may help in the reduction of breast cancer cell proliferation, causing apoptosis in different stages depending on the type of preparation.
Keywords
Urtica urens, MCF-7, Breast cancer, Apoptosis, ROS, Cell cycle arrest, Plant extract, Flow cytometry, Apoptosis, Cell cycle, ROS, Complementary and Alternative Medicine (CAM)
Introduction
Herbal extracts are widely used to reduce or cure many ailments worldwide. Among them, nettles are used and sold on a large scale. Urtica or stinging nettle, belongs to the Urticaceae family and occurs as a perennial plant in temperate zones (Bisht, et al. 2012). It grows in Europe (Vogl and Hart, 2003), the Middle East and North and South America (Rodriguez-Fragoso, 2008, Woodland, et al. 1976). It thrives in wet, nitrogen-rich soil and grows naturally (Kumar, et al. 2013). Both types of stinging nettle, Urtica urens and Urtica dioica, are approved for use in many European countries as liquid and dry extracts (European Medicines Agency, 2012, Viotti, et al. 2024). Urtica dioica leaf extract has shown anti-nociceptive and anti-inflammatory effects (Hajhashemi and Klooshani, 2012, Abi Sleiman, et al. 2024). Urtica dioica has demonstrated antihepatotoxic effects by reducing oxidative stress and lipid peroxidation in liver tissues, thus protecting the liver from various forms of damage in animal models (Gao, et al. 2024), and has chemoprotective abilities in other animal models (Abi Sleiman, et al. 2024). The above-ground parts are also used for allergic rhinitis (Bakhshaee, et al. 2017), osteoarthritis (Randall, 1994) and diabetes (Chehri, et al. 2022). The aqueous extract has been found to inhibit prostate (Abi Sleiman, et al. 2024) and breast cancer in human tissues (Fattahi, et al. 2013). Additionally, the root extract is used for urination problems related to an enlarged prostate, known as benign prostatic hyperplasia (Safarinejad, et al. 2005). The methanolic extract of stinging nettle root has also shown antiproliferative effects on human prostate cancer cells (Abi Sleiman, et al. 2024). Urtica urens can be found in Saudi Arabia within the local flora and is noted for its medicinal and nutritional benefits. Research indicates that the leaves of Urtica urens are rich in bioactive compounds that possess antioxidant and anti-inflammatory effects, which aligns with its traditional applications in treating infections and various ailments. Pharmacological assessments reveal substantial antinociceptive and anti-inflammatory actions of Urtica urens extracts, emphasising their potential for therapeutic use (Taheri, et al. 2022).
Breast cancer is one of the most common cancers globally. Urtica urens is a popular complementary and alternative medicine for cancer patients. Urtica urens extracts have significant cytotoxicity against MCF-7 cells. These findings highlight Urtica urens's potential as a source of bioactive compounds with anticancer properties, warranting further investigation into its mechanisms and therapeutic applications in breast cancer (Gaafar, et al. 2020). While Urtica urens has traditionally been used in complementary and alternative medicine for various conditions, including cancer, there is still limited research on its cytotoxic effects on MCF-7 breast cancer cells. Al Doghaither et al. (2016) notably found that aqueous extracts of Urtica urens displayed moderate cytotoxicity against MCF-7 cells; however, the mechanisms behind this action, including Reactive Oxygen Species (ROS) generation, apoptosis progression and cell cycle effects, have yet to be fully clarified. A person with cancer or willing to prevent cancer can try every medicine to save their life. It is expected that 26 million new cancer cases and 17 million deaths per year will occur by 2030 (Solowey, et al. 2014). In Turkey, almost 93% of cancer patients have used nettle herbs as Complementary and Alternative Medicine (CAM) (Göz±m et al., 2003). In the USA, there was a high prevalence of using CAM among women at risk of breast cancer (Greenlee, et al. 2016). In the USA, CAM use is also highly prevalent among women with breast cancer and those at risk, with recent data showing that 84% of breast cancer patients reported using some form of CAM during treatment (Ryan, et al. 2022). However, some Complementary and Alternative Medicine (CAM) may have cytotoxic effects on cells. The use of CAM on cancer cells has been reported in many studies with positive outcomes (Rockwell, et al. 2005; Liao, et al. 2005). Another warning study by Wojcikowski, et al. (2004) noted increased renal cytotoxicity among CAM users. Accordingly, further investigation must be done to confirm the effects of such widely used herbs.
Previous studies of Emmelin and Feldberg (1947) showed the effect of the active substances and the reaction of the fluid of the nettle hair on the human skin and tongue (Caliskaner, et al. 2004). While most studies on Urtica urens have focused on testing anti-inflammatory effects (Arslan, et al. 2014), on rats (Ozkarsli, et al. 2008) and antioxidant effects (Kumar, et al. 2013).
Measuring cancer cells' proliferation after drug treatment is a significant technique for detecting their effects. Apoptosis is a mechanism in which cells inhibit cancer cells and is associated with changes in Reactive Oxygen Species (ROS) (Trachootham, et al. 2009). Reactive oxygen species include several molecules that damage DNA and RNA and oxidise proteins and lipids (lipid peroxidation). These reactive molecules contain an oxygen and include H2O2 (Hydrogen Peroxide), NO (Nitric Oxide), O2- (Oxide anion), Peroxynitrite (ONOO-), Hydrochlorous Acid (HOCl) and Hydroxyl Radical (OH-). Oxidative species are produced in pathological situations; for example, the superoxide radical is generated as a by-product of oxidative phosphorylation in living cells.
This study aims to determine the apoptotic potential of the leaves of Urtica urens (dwarf nettle) on cultured human breast cancer cells (MCF-7). The water extract (2 mg/ml) of both the fresh and the freeze-dried leaves was used to evaluate the apoptotic effect and the morphological changes of the cancer cells. The apoptotic type and cell cycle changes were determined using Annexin V and Propidium Iodide (PI) for both extracts. At the same time, mitochondrial and cytoplasmic ROS were analysed using flow cytometry.
Materials and Methods
Cell samples
The malignant (MCF-7) cells were obtained from the Tissue Culture Bank at King Fahad Medical Research Centre, King Abdulaziz University in Jeddah, Saudi Arabia. Cells were grown in Dulbecco’s Modified Eagle Medium (DMEM) supplemented with 10% fetal bovine serum, 2 mM glutamine and 1% penicillin and streptomycin (Sigma-Aldrich, St. Louis, MO). They were cultured in 75 cm² tissue culture flasks (Corning, USA) at a density of 8 × 105 to approximately 60%–70% confluency.
Extract preparation
Urtica urens was purchased in a single batch from a local organic shop in Jeddah, Saudi Arabia. The plant was collected from the Al-Taif West region of the country. The purpose of choosing this extraction method is to closely resemble how most people consume the herb by soaking it, rather than using alcohol extraction. First, a freeze-dried powder extract was prepared by boiling 50 g in 1500 ml of distilled water for 30 minutes. The liquid was filtered through gauze and the residues were washed several times with distilled water. The collected liquid was freeze-dried, yielding 9 g of powder. On the day of the experiment, stock solutions of freeze-dried Urtica urens extract were prepared by dissolving 0.4 g in 100 ml of PBS containing Ca2+ and Mg2+ and stirred for 30 minutes. Simultaneously, the dry leaves extract, prepared in Turkish folk (personal communication), was made by soaking 0.4 g of the dry herb in 100 ml of boiled water. Immediately after preparation, both powder extracts and the dry tea extract were centrifuged at 1500 rpm for 10 minutes. The supernatant was sterilised using a 0.22 μm filter before being added to the culture medium at a ratio of 1:1 to create a final concentration of 2 mg/ml, referred to as treatment. Before each experiment, the culture medium was discarded, and both extract treatments of Urtica urens (10 ml) were added and incubated for 24 hours.
Morphology changes
After culturing and seeding MCF-7 cells in 75 cm² flasks at a concentration of 8 × 105 cells/ml, treatments were added as mentioned above. Morphological changes were observed using a phase contrast inverted microscope (TH4-200, Olympus Optical Co. Ltd, Japan), with untreated cells as a control.
Cell viability by trypan blue
Cell count assay of MCF-7 and control cells was performed using a hemocytometer slide and trypan blue dye (Hyclone, Logan, USA) as described by Mather & Roberts, 1998. MCF-7 cells were seeded in 75 cm² flasks at 8 × 105 cells/ml. After treatments, cell pellets were suspended in 1 ml of culture medium and diluted 1:1 with 0.4% trypan blue dye. Live cells were counted and the average was calculated using the following equation:
Cell count/ml=dilution factor × average count × 104
Cell viability by MTT
The yellow colour of tetrazolium salt 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT, Sigma-Aldrich Chemical Co., UK) is converted by viable cells to purple colored formazan crystals. Cells were seeded into 96-well tissue culture plates in 200μl culture medium containing 1 × 104 cells/well. Culture medium was aspirated and 200 μl of the treatments diluted (1:1) with medium was added. After 24 h, 20 μl of MTT (5 mg/ml) was added to each well according to Korashy, et al. (2012) and incubated for 2 hours. Medium was then aspirated and replaced with 200 μl of isopropyl. The produced crystals were dissolved by shaking the plate for 30 minutes at room temperature. The resultant purple colour was measured at 570 nm. Mean values ± SD for each concentration were determined. Cell viability (in percentages) was shown as the ratio of absorbance (A 570 nm) in treated cells relative to absorbance in control cells.
Cell viability (%) = (A570 nm (Sample)/A570 nm (Control)) × 100
Cytoplasmic Reactive Oxygen Species (ROS)
The intracellular ROS levels in MCF-7 cells treated with 2 mg/ml Urtica urens were measured by flow cytometry using non-fluorescent carboxy-2′,7′-dichlorodihydrofluorescein diacetate (carboxy-H2DCFDA) (Invitrogen, UK). This dye is converted into a fluorescent derivative when reacting with ROS, mainly H2O2. Cells were seeded in 75 cm2 flasks at 8 × 105 cells/ml. Treatments were applied once cells reached 60%-70% confluence. Per the manufacturer’s instructions, cells were incubated with 5 μM H2DCFDA for 30 min at 37°C and 5% CO2. They were kept on ice under low light conditions until carboxy-H2DCF fluorescence was measured using a Becton-Dickinson (BD) FACSCanto flow cytometer (California, USA). At least 10,000 events were acquired in the gated regions using an emission wavelength of 520 nm.
Mitochondrial Reactive Oxygen Species (ROS)
Red fluorogenic dye, Mitosox (Invitrogen, UK), is a selective dye for the mitochondria's superoxide free radical (O2-). The O2-. levels in control MCF-7 and treated cells with 2 mg/ml Urtica urens were measured by flow cytometry according to the manufacturer’s instructions. Briefly, treated cells were washed with 1 ml of PBS (1X) and re-suspended in 500 μl buffer. Mitosox dye (5 μl) was added and incubated for 10 minutes in the dark. The red fluorescence was measured using a BD FACSCanto flow cytometer (California, USA). At least 10,000 events were acquired in the gated regions using an emission wavelength of 620 nm.
Apoptosis by Annexin-V with Propidium Iodide (PI)
Annexin V-FITC with PI kit purchased from Abcam, UK, was used to detect early and late apoptosis and necrosis in MFC-7 cells treated with 2 mg/ml freeze-dried and fresh-dried leaves of Urtica urens. Annexin V binds phosphatidylserine that has been externalised from the inner surface of the cell membrane to the outer surface during early apoptosis. Propidium Iodide (PI) was also used to distinguish between the necrotic and apoptotic cells. Early apoptotic cells will exclude PI, while late-stage apoptotic cells and necrotic cells will stain positively, due to the passage of these dyes into the nucleus where they bind to DNA. Cells were seeded in 75 cm² flasks with a density of 8 × 105 cells/ml. Until 60%-70% confluency. After 24 h of treatments, floating and adherent cells were washed with 1 ml of PBS (Oxoid, Hampshire, UK) and suspended in 500 μl of 1x cold binding buffer. Annexin V-FITC and PI (5 μl) were added to each flow tube according to the kit instructions. Cells were incubated for 5 minutes in the dark at room temperature. A FACSCanto flow cytometer (California, USA) was used for measurements. A minimum of 10,000 events were acquired in the gated regions. Annexin V-FITC-labelled and PI-labelled emissions were 520 and 620 nm, respectively.
Cell cycle
Staining cells’ DNA with PI is a classic means of cell cycle analysis. MCF-7 cells were seeded in 75 cm2 flasks at 8 × 105 cells/ml. Treatments with Urtica urens were added to the cells. After trypsinisation, cells were suspended in 200 μl cold PBS, fixed with 1 ml of cold fixing buffer (70% ethanol in PBS), and incubated at 4°C. After 24 h, cells were washed twice with PBS and suspended in 500 μl of PBS, followed by adding 5 μl of RNase (1 mg of RNase/8 ml PBS). A further incubation period of 30 min at 37°C was followed by adding 5 μl of PI (1 mg/ml). Cells were kept on ice in dark conditions until analysed by a BD FACSCalibur flow cytometer. At least 10,000 events were acquired in the list mode using an emission wavelength of 620 nm.
Statistical analysis
All experiments were performed in triplicate. GraphPad Prism 6 software was used for statistical analysis. Significant differences between the control and the experimental values were evaluated using P-values determined by one-way Analysis of Variance (ANOVA), followed by Bonferroni corrections. The significance level was ≤ 0.05.
Results
Morphology changes
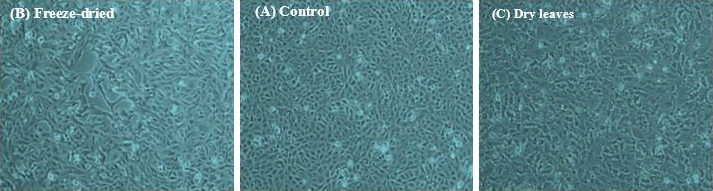
It was found that the morphology of the MCF-7 cells changed upon treatment with 2 mg/ml of both freeze-dried and fresh-dried leaf extracts (Fig. 1). Untreated control cells (A) appeared intact with a complete sheet and no signs of apoptosis. After adding 2 mg/ml freeze-dried leaves (B) and fresh-dried leaf extracts (C), cells started to round up and detach from the plate, forming vacuoles in the sheet.
Figure 1: Morphology of MCF-7 cells incubated in MEM supplemented with 10% FBS and a final concentration of 2 mg/ml Urtica urens for 24 hours. A is an untreated control. B is a freeze-dried extract. C is fresh, dry leaves. A Nikon Eclipse microscope at 10x magnification was used.
Cell viability by Trypan blue and MTT
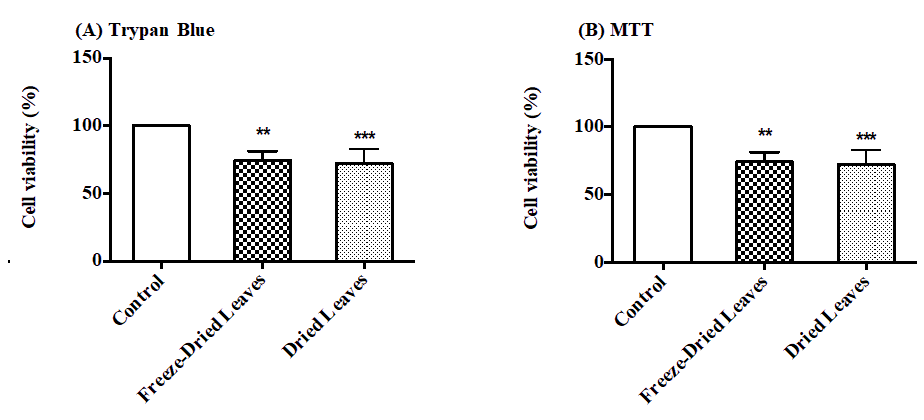
Both extracts showed a significant reduction (p ≤ 0.05) in cell viability in MCF-7 treated with 2 mg/ml Urtica urens. The viability dropped from 100 ± 1.2% to 74.35 ± 7.1 and 72.13 ± 10.76% for freeze-dried and dried leaves when estimated by trypan blue (Fig. 2A). The same reduction was confirmed when cell viability was measured with MTT (Fig. 2 B). Again, it was reduced to 69.47% and 71.86%, respectively.
Figure 2: The effect of Urtica urens 2 mg/ml incubated with MCF-7 cells for 24 h on cell viability estimated by Trypan blue (A) and MTT (B) (*) indicates statistically significant differences between controls and treatments (**p<0.05; ***p<0.01)..
Cytoplasmic Reactive Oxygen Species (ROS)
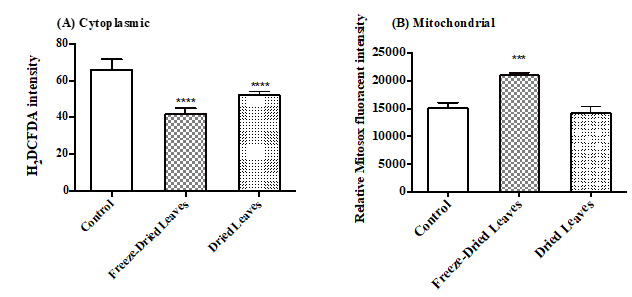
The intensity of fluorescent H2DCFDA represents the intracellular ROS production. Both treatments significantly decreased ROS compared to control cells (Fig. 3). Freeze-dried treated cells showed a greater reduction from 66 ± 5.5 to 41.83 ± 3.1 compared to 52.1 ± 1.9 caused by fresh dried leaves extract. These values were expressed as the means ± SEM of at least three independent experiments.
Figure 3: The effect of freeze-dried and fresh dry leaves extracts of Urtica urens (2 mg/ml) on the production of ROS in MCF-7 cells treated for 24 h. (A) Cytoplasmic ROS production, the intensity of H2DCFDA, was measured by BD FACSCanto flow cytometer at 520 nm. (B) Mitochondrial ROS production was measured by Mitosox red fluorogenic intensity at 620 nm BD FACSCanto flow cytometer. Note:These values were expressed as means ± SEM of at least three independent experiments (***p<0.05; ****p<0.01).
Mitochondrial Reactive Oxygen Species (ROS)
The intensity of the red Mitosox dye is proportional to mitochondrial ROS production (Fig. 3B). When MCF-7 cells were treated with freeze-dried leaves of Urtica urens, ROS production significantly increased from 15037 ± 1066 to 20973 ± 488, with no significant effect observed when fresh dried leaves extract was used (14140 ± 1301).
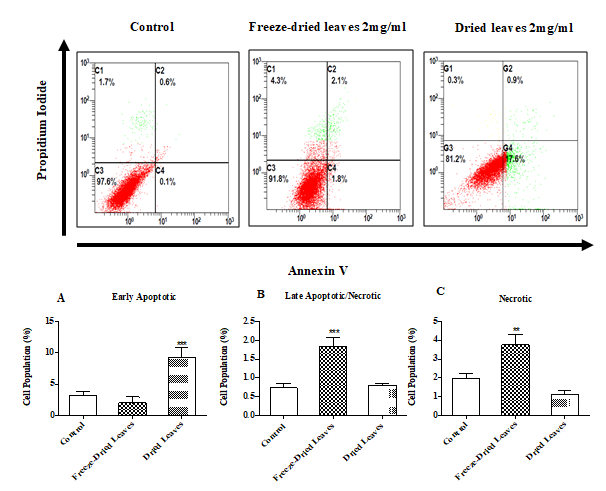
Apoptosis detection by Annexin-V
The two extracts caused cell death and the mechanisms by which these extracts caused such death displayed different patterns (Fig. 4 A, B, and C). Freeze-dried leaves show a significant increase in the late apoptosis and necrosis phase compared to the early apoptosis population when cells were treated with fresh dried leaf extracts.
Figure 4: The apoptosis mechanism of two Urtica urens extracts incubated for 24 h with MCF-7 cells. Different cell populations were designated as Annexinv −ve and PI−ve=viable cells; Annexinv +ve and PI −ve=apoptotic cells and Annexinv +ve and PI +ve=late apoptotic or necrotic cells. Beckman FACSCanto flow cytometry (USA) was used for analysis. Note: Values were expressed as means ± SEM of at least three independent experiments (**p<0.05; ***p<0.01).
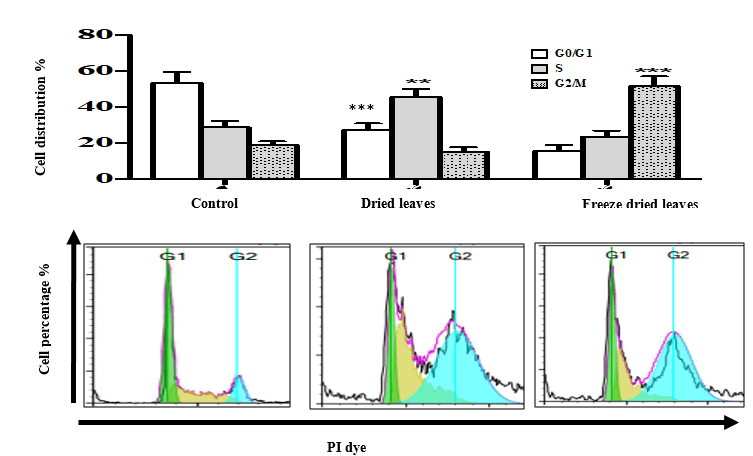
Cell cycle
The effects of both water extracts of Urtica urens (2 mg/ml) on the MCF-7 cell cycle are shown in Fig. 5. Both caused a significant decrease in the G0/G1 population after 24 hours (P ≤ 0.001). This decrease was from 53.37% in controls to 27.5% and 15.6% for freeze-dried leaves and fresh-dried leaves, respectively. The reduction in cell population was associated with a significant increase in the S phase for cells treated with freeze-dried extract, showing an increase of 16.7%, compared to a 32.4% increase in the G2/M phase for cells treated with the dried leaves extract.
Figure 5: Cell cycle arrest of MCF-7 treated with 2 mg/ml Urtica urens extracts, incubated for 24 h. Following drug treatments, the cells were harvested by trypsinisation, collected by centrifugation, re-suspended and fixed in ethanol, and incubated at 20°C. Cells were then stained with PI for 30 min and cell cycle distribution was determined using a FACScan flow cytometer with CellQuest software. Note: Results were expressed as mean ± SEM of at least three independent experiments (**p<0.05; ***p<0.01).
Discussion
The search for plant-based anticancer agents remains a central theme in oncology research, especially as challenges like multi-drug resistance and treatment-related toxicity undermine the effectiveness of conventional chemotherapy. In this regard, Urtica urens stands out as a potential option due to its longstanding use in traditional medicine and emerging evidence indicating its antioxidant, anti-inflammatory and antitumor properties (Gao, et al. 2024, Kregiel, et al. 2018). Nonetheless, there is a lack of thorough mechanistic research on Urtica urens in the current literature, specifically concerning its varied effects linked to different extraction techniques and its role in modulating oxidative stress in breast cancer cells. This study seeks to fill these gaps by exploring the cytotoxic and mechanistic impacts of aqueous extracts of Urtica urens, derived through freeze-drying and fresh-drying methods, on MCF-7 human breast adenocarcinoma cells.
Cytotoxic effects and morphological evidence of apoptosis
Our results show that both Urtica urens extracts significantly reduced MCF-7 cell viability, accompanied by typical apoptotic morphological features such as cell shrinkage, membrane blebbing and detachment. These findings are consistent with prior work on Urtica dioica, where dose-dependent cytotoxicity in MCF-7 cells was reported, although those studies lacked mechanistic follow-up (Fattahi, et al. 2013, Sadeghi, et al. 2021).
ROS as a key regulator of cell fate: A redox switch mechanism
A significant finding of this study is the dual modulation of intracellular ROS by Urtica urens extracts. Both preparations significantly reduced cytoplasmic ROS, potentially due to antioxidant phytochemicals. In contrast, only the freeze-dried extract elevated mitochondrial ROS, suggesting activation of the intrinsic apoptotic pathway. Mitochondrial ROS triggers cytochrome c release and caspase activation, initiating programmed cell death (Trachootham, et al. 2009, Yang and Yi, 2008). This biphasic ROS pattern aligns with the “oxidative window” hypothesis, where moderate mitochondrial ROS promotes apoptosis, while elevated cytoplasmic ROS may instead trigger adaptive survival mechanisms. The distinct ROS responses may reflect alterations in phytochemical stability due to the drying method (Gaafar, et al. 2020, Kregiel, et al. 2018).
Distinct apoptotic pathways and cell cycle effects based on extract type
Flow cytometry revealed that Urtica urens induces apoptosis through distinct mechanisms depending on extract type. The fresh-dried extract favoured early apoptosis, while the freeze-dried extract led to late apoptosis and necrosis, likely due to mitochondrial oxidative stress. Correspondingly, the extracts induced different cell cycle arrest phases: freeze-dried extract led to S phase accumulation, and the fresh-dried extract induced G2/M arrest. These differences are consistent with findings from other plant-based compounds such as Phyla nodiflora and Myoporum bontioides, which also interfere with DNA synthesis or mitotic progression through modulation of cyclins and CDKS (Teoh, et al. 2019, Weng, et al. 2017).
Hypothesised molecular pathways
Although molecular markers were not directly measured, the observed ROS profiles and apoptosis patterns suggest activation of well-established cancer signalling pathways. Mitochondrial ROS elevation by the freeze-dried extract likely initiates the intrinsic apoptotic pathway, involving Bax/Bcl-2 regulation, cytochrome c release and caspase-9/3 activation. In contrast, early apoptosis induced by the fresh-dried extract may involve p53, a redox-sensitive transcription factor that coordinates DNA damage responses and apoptotic gene expression (Kroemer and Reed, 2000, Vousden and Lane, 2007, Green and Kroemer, 2004).
Regarding cell cycle regulation, S phase arrest may result from upregulation of p21 and inhibition of cyclin A/CDK2 complexes, while G2/M arrest may involve suppression of cyclin B1/CDK1 activity or ATM/Chk1 checkpoint activation. Polyphenols and flavonoids frequently modulate these pathways-bioactive compounds abundant in Urtica species-supporting the plausibility of our proposed mechanism.
Hypothesised molecular pathways related to Urtica urens cytotoxicity
Although this study did not directly evaluate molecular markers associated with apoptosis or cell cycle regulation, the observed patterns such as mitochondrial ROS elevation, phase-specific cell cycle arrest and distinct stages of apoptosis-strongly suggest the involvement of well-established signalling pathways in cancer cell fate regulation.
The significant rise in mitochondrial ROS following treatment with freeze-dried Urtica urens extract likely compromises mitochondrial membrane integrity, leading to the release of cytochrome c and activation of the intrinsic apoptotic pathway via caspase-9 and the downstream effector caspase-3. Bcl-2 family proteins typically govern this mitochondrial stress and the extract may promote apoptosis by downregulating anti-apoptotic Bcl-2 and upregulating pro-apoptotic Bax, thereby shifting the balance toward cell death. Meanwhile, early apoptosis induced by the fresh-dried extract, along with the observed reduction in cytoplasmic ROS, may involve activation of p53, a redox-sensitive tumour suppressor that orchestrates DNA damage responses and early apoptotic signalling (Kroemer and Reed, 2000, Vousden and Lane, 2007, Green and Kroemer, 2004, Chipuk, et al. 2006).
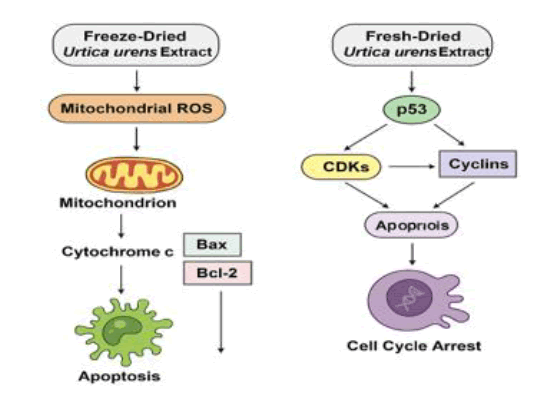
Regarding cell cycle control, the S phase arrest observed with freeze-dried extracts may reflect interference with DNA replication checkpoints, potentially mediated by p21 upregulation and inhibition of cyclin A/CDK2 complexes. Conversely, G2/M arrest induced by the fresh-dried extract may involve suppressing cyclin B1/CDK1 activity or activating the G2 DNA damage checkpoint through ATM/Chk1 signalling. Polyphenols and flavonoids found in medicinal plants, including Urtica species, frequently target these molecular pathways, supporting the proposed mechanisms' plausibility (Fig. 6).
Figure 6: Proposed molecular pathways illustrating the cytotoxic effects of freeze-dried and fresh-dried Urtica urens extracts on MCF-7 cells. Freeze-dried extracts are proposed to elevate mitochondrial ROS, triggering intrinsic apoptosis via Bax/Bcl-2 and caspase activation. Fresh-dried extracts may act through p53-mediated signalling, leading to early apoptosis and G2/M arrest.
Conclusion
This study offers new insight into the cytotoxic mechanisms of Urtica urens aqueous extracts in breast cancer cells, emphasising how the extract preparation method strongly influences biological activity. Freeze-dried extracts induced late apoptosis and mitochondrial ROS accumulation, while fresh-dried extracts favoured early apoptosis and cytoplasmic ROS reduction. Each preparation type also caused phase-specific cell cycle arrest, reinforcing the link between phytochemical integrity and cellular outcome. These findings suggest that Urtica urens may serve as a redox-sensitive therapeutic candidate for breast cancer treatment.
Future Directions
To further validate these findings and support translational application, we recommend the following:
•Phytochemical profiling (e.g., HPLC, LC-MS) to identify key bioactive compounds.
•Gene and protein expression analysis (e.g., Bcl-2, Bax, caspases, cyclins) to confirm pathway activation.
•3d tumour models and co-culture systems to better reflect in vivo behaviour.
•In vivo studies to assess efficacy, pharmacokinetics and toxicity in animal models.
•Combination therapies to evaluate whether Urtica urens enhances or synergises with chemotherapeutic agents. This study establishes a mechanistic basis for repositioning Urtica urens as a scientifically recognised anticancer agent, enhancing its transition from traditional herbal usage to contemporary therapeutic application.
Conflict of Interest
The authors have no conflicts of interest relevant to this article to disclose.
Disclosure Summary
The authors have nothing to disclose. This manuscript describes original work and is not under consideration by any other journal.
References
- Bisht S, Bhandari S, Bisht NS. (2012). Urtica dioica (L): An undervalued, economically important plant. Agric Sci Res J. 2:250-252.
- Vogl R, Hart A. (2003). Production and processing of organically grown fiber nettle (Urtica dioica L.) and its potential use in the natural textile industry: A review. Am J Alternative Agric. 18:119-128.
- RodriguezâFragoso L, ReyesâEsparza J, Burchiel W, HerreraâRuiz D, Torres E. (2008). Risks and benefits of commonly used herbal medicines in Mexico. Toxicol App Pharmacol. 227:125-135.
[Crossref] [Google Scholar] [PubMed]
- Woodland W, Bassett J, Crompton W. (1976). The annual species of stinging nettle (Hesperocnide and Urtica) in North America. Canadian J Bot. 54:374-383.
- Kumar HM, Prathima VR, Sowmya S, Siddagangaiah S, Thribhuvan KR. (2013). Study of nutritional quality, phytochemical constituents and antioxidant activities by different solvents of nettle (Urtica urens) from Madikeri-Karnataka State. Int Res J Pharm App Sci. 3:112-119.
- European Medicines Agency. (2012). Assessment report on Urtica dioica L., Urtica urens L., their hybrids or their mixtures, radix.
- Viotti C, Bertheau C, Martz F, Yung L, Placet V, Ferrarini A, Fornassier F, Blaudez D, Puschenreiter M, Chalot M. (2024). Digestate improves stinging nettle (Urtica dioica) growth and fiber production at a chlorâalkali site. Plants. 13:2425.
[Crossref] [Google Scholar] [PubMed]
- Hajhashemi V, Klooshani V. (2013). Antinociceptive and anti-inflammatory effects of Urtica dioica leaf extract in animal models. Avicenna J Phytomed. 3:193.
[Google Scholar] [PubMed]
- Abi Sleiman M, Younes M, Hajj R, Salameh T, Abi Rached S, Abi Younes R, Daoud L, Doumiati JL, Frem F, Ishak R, Medawar C, Naim HY, Rizk S. (2024). Urtica dioica: Anticancer properties and other systemic health benefits from in vitro to clinical trials. Int J Mol Sci. 25:7501.
[Crossref] [Google Scholar] [PubMed]
- Gao Y, Yang X, Chen B, Leng H, Zhang J. (2024). The biological function of Urtica spp. and its application in poultry, fish and livestock. Front Vet Sci. 11:1430362.
[Crossref] [Google Scholar] [PubMed]
- Bakhshaee M, Esmaeili M, Azad FJ, Talesh GA, Salehi M, Mohajer MN. (2017). Efficacy of supportive therapy of allergic rhinitis by stinging nettle (Urtica dioica) root extract: A randomized, double-blind, placebo-controlled, clinical trial. Iran J Pharm Res. 16:112.
[Google Scholar] [PubMed]
- Randall CF. (1994). Stinging nettles for osteoarthritis pain of the hip. British J of Gen Pract. 44:533-534.
- Chehri A, Yarani R, Yousefi Z, Novin Bahador T, Shakouri SK, Ostadrahimi A, Mobasseri M, Pociot F, Araj-Khodaei M. (2022). Anti-diabetic potential of Urtica dioica: Current knowledge and future direction. J Diabetes Metab Disord. 21:1.
[Crossref] [Google Scholar] [PubMed]
- Fattahi S, Ardekani AM, Zabihi E, Abedian Z, Mostafazadeh A, Pourbagher R, Akhavan-Niaki H. (2013). Antioxidant and apoptotic effects of an aqueous extract of Urtica dioica on the MCF-7 human breast cancer cell line. Asian Pac J Cancer Prev. 14:5317-5323.
[Crossref] [Google Scholar] [PubMed]
- Safarinejad M. 2005. Urtica dioica for treatment of benign prostatic hyperplasia: A prospective, randomized, doubleâblind, placeboâcontrolled, crossover study. J Herbal Pharmacother. 5:1-11.
- Taheri Y, Quispe C, HerreraâBravo J, SharifiâRad J, Ezzat SM, Merghany RM, Shaheen S, Azmi L, Prakash Mishra A, Sener B, Kılıç M, Sen S, Acharya K, Nasiri A, CruzâMartins N, Tsouh Fokou PV, Ydyrys A, Alibek K, Bassygarayev Z, DaÅtan SD, Alshehri MA, Al Doghaither HA, Omar UM, Rahimulddin SA, AlâGhafari AB. (2016). Cytotoxic effects of aqueous extracts of Urtica urens on most common cancer types in Saudi Arabia. J Biol Sci. 16:242-246.
- Gaafar AA, Ali SI, Kutkat O, Kandeil AM, El-Hallouty SM. (2020). Bioactive ingredients and anti-influenza (H5N1), anticancer and antioxidant properties of Urtica urens L. Jordan J Biol Sci. 13:647-657.
- Solowey E, Lichtenstein M, Sallon S, Paavilainen H, Solowey E, LorberboumâGalski H. (2014). Evaluating medicinal plants for anticancer activity. EvidenceâBased Compl Alter Med. 2014:721402.
[Crossref] [Google Scholar] [PubMed]
- Gözüm S, Tezel A, Koc M. (2003). Complementary alternative treatments used by patients with cancer in eastern Turkey. Cancer Nurs. 26:230-236.
[Crossref] [Google Scholar] [PubMed]
- Greenlee H, Sardo Molmenti CL, Falci L, Ulmer R, Deming-Halverson S, DeRoo LA, Sandler DP. (2016). High use of complementary and alternative medicine among a large cohort of women with a family history of breast cancer: The sister study. Breast Cancer Res Treat. 156:527-538.
[Crossref] [Google Scholar] [PubMed]
- Rockwell SL, Higgins S. (2005). Alteration of the effects of cancer therapy agents on breast cancer cells by the herbal medicine black cohosh. Breast Cancer Res Treat. 90:233-239.
[Crossref] [Google Scholar] [PubMed]
- Liao C, Pan S, Guh J, Chang Y, Pai H, Lin C, Teng C. (2005). Antitumor mechanism of evodiamine, a constituent from Chinese herb Evodiae fructus, in human multipleâdrug resistant breast cancer NCI/ADRâRES cells in vitro and in vivo. Carcinogenesis. 26:968-975.
[Crossref] [Google Scholar] [PubMed]
- Wojcikowski K, Johnson W, Gobé G. (2004). Medicinal herbal extracts-renal friend or foe? Part one: The toxicities of medicinal herbs. Nephrology. 9:313-318.
[Crossref] [Google Scholar] [PubMed]
- Enunelin N, Feldberg W. (1947). The mechanism of the sting of the common nettle (Urtica dioica). J Physiol. 106:440-445.
- Caliskaner Z, Karaayvaz M, Ozturk S. (2004). Misuse of a herb: Stinging nettle (Urtica urens) induced severe tongue oedema. Complement Ther Med. 12:57-58.
[Crossref] [Google Scholar] [PubMed]
- Arslan S, Terzioglu G, Elcil S, Deligoz H, Sen A. (2014). Assessing of anti-inflammatory effect of small nettle' (Urtica urens) increasing polarity extracts. J Neuroimmunol. 275:135.
- Ozkarsli HS, Sevim H, Sen A. (2008). In vivo effects of Urtica urens (dwarf nettle) on the expression of CYP1A in control and 3âmethylcholanthreneâexposed rats. Xenobiotica. 38:48-61.
[Crossref] [Google Scholar] [PubMed]
- Kumar HM, Prathima VR, Sowmya S, Siddagangaiah S, Thribhuvan KR. (2013). Study of nutritional quality, phytochemical constituents and antioxidant activities by different solvents of nettle (Urtica urens) from Madikeri-Karnataka State. Intern J Pharmaceu Sci Res. 4:3820–3824.
- Trachootham D, Alexandre J, Huang P. (2009). Targeting cancer cells by ROSâmediated mechanisms: A radical therapeutic approach? Nat Rev Drug Discovery. 8:579-591.
[Crossref] [Google Scholar] [PubMed]
- Mather JP, Roberts PE. (1998). Introduction to cell and tissue culture: Theory and technique. Springer Science Business Media.
- Kregiel D, Pawlikowska E, Antolak H. (2018). Urtica spp.: Ordinary plants with extraordinary properties. Molecules. 23:1664.
[Crossref] [Google Scholar] [PubMed]
- Sadeghi N, Mahdavi M, Abadi A, Farhadi F. (2021). Apoptotic and cell cycle arrest effects of Urtica dioica extract on MCFâ7 cells. Biomed Pharmacother. 134:111125.
- Yang J, Yi Q. (2008). Redox regulation and cancer treatment: The role of ROS in cancer chemotherapy. Curr Med Chem. 15:302-311.
- Teoh PL, Liau M, Cheong B. (2019). Phyla nodiflora L. extracts induce apoptosis and cell cycle arrest in human breast cancer cell line, MCFâ7. Nutrition and Cancer.71:1-8.
[Crossref] [Google Scholar] [PubMed]
- Weng JR, Bai LY, Lin WY, Chiu CF, Chen YC, Chao SW, Feng CH. (2017). A flavone constituent from Myoporum bontioides induces Mâphase cell cycle arrest of MCFâ7 breast cancer cells. Molecules. 22:472.
[Crossref] [Google Scholar] [PubMed]
- Kroemer G, Reed JC. (2000). Mitochondrial control of cell death. Nat Med. 6:513-519.
[Crossref] [Google Scholar] [PubMed]
- Vousden KH, Lane DP. 2007. p53 in health and disease. Nature Rev Molec Cell Biol. 8:275-283.
[Crossref] [Google Scholar] [PubMed]
- Green DR, Kroemer G. (2004). The pathophysiology of mitochondrial cell death. Science. 305:626-629.
[Crossref] [Google Scholar] [PubMed]
- Chipuk JE, Bouchier-Hayes L, Green DR. (2006). Mitochondrial outer membrane permeabilization during apoptosis: The innocent bystander scenario. Cell Death Differ. 13:1396-1402.
[Crossref] [Google Scholar] [PubMed].