Research Article - Modern Phytomorphology ( 2020) Volume 14, Issue 2
Study the mercury biosorption by unicellular diatom Nitzschia capitellata Hustedt
Ali Naseri1*, Sara Saadatmand1, Mostafa Norozi2, Younes Asri3 and Alireza Iranbakhsh12Departments of Biotechnology, University of Alzahra, Iran
3Agricultural Research, Education and Extension Organization (AREEO), Research Institute of Forests and Rangelands, Tehran, Iran
Ali Naseri, Department of Biology, Science and Research Branch, Islamic Azad University, Tehran, Iran, Email: sadatmandsara@gmail.com
Received: 28-Feb-2020 Accepted: 29-Apr-2020 Published: 06-May-2020, DOI: 10.5281/zenodo.5077883
Abstract
Present study was conducted to investigate the ability of Nitzschia capitellata in accumulation of heavy metal, together with investigating the concentration of the heavy metals absorbed by N. capitellata. The diatom samples of the study collected from Taleghan River in the west of Alborz Province, Iran. The isolated and purified diatom was transferred to the liquid culture medium (F/2) and Mercury test concentrations were 90, 285 and 300 μg/L of HgCl2 in three replications. Cold Atomic Absorption Spectrometry method was used to measure mercury concentration. The precipitation of 30 ml of diatoms’ culture with cell density of 350 × 103 cell/cm2 was added to each mercury concentration. After inoculation, the DTE 0.01 molL-1 solution was used to separate the mercury bonded to the cell wall. The N. capitellata cells were killed when they were exposed to 1000 μgL-1 HgCl2 concentration. The highest intracellular absorbance (416.50 μgL) was related to the first day of treatment in 285 μgL-1 concentration of mercury, and the highest discharge of mercury to environment was 256 μgL-1 concentration in the fourth day. and also the concentrations of intracellular solution mercury and bonded mercury was significantly different.
Keywords
Nitzschia capitellata, detoxification, heavy metal, mercury biosorption
Introduction
The algae have unique characteristic, due to the fact that algae, particularly marine algae, are relatively able to accumulate high metal content. These structures are high resistance and have ability to grow in autotroph and heterotrophic condition. They have the metal storing factors, ability to heavy metals compartmentation, high surface/volume ratio, and able to phytochelation.
Using algae has been one of the most important ways in purification of water and industrial wastewater supplies in recent years. The presence of heavy metals is increasing Due to natural reasons as volcanic activities and some other unnatural reasons caused by Human such as mining, burning fossil fuels and metal industries (Jain 2004). According to Saadatmand & Niazi (2015), 53 out of 90 known chemical elements are considered as heavy metals with a density of more than 5 gcm-3. They also are derived from renewal of natural soils because of the changes in local redox conditions and the corrosion of subsurface engineering structures due to continued immersion under acidic groundwater (Leung & Jiao 2006). Mercury enters the environment as a result of normal breakdown of minerals in rocks and soil through exposure to wind and water. Release of mercury from natural sources has remained fairly the same over the years. Accordingly, still mercury concentrations in the environment as one of the typical toxic heavy metals, are increasing; this is ascribed to human activity and also is a global problem because of its persistence, bioaccumulation and toxicity in the environment (Deng etal. 2013; Wilde & Benemann 1993). Recently the studies of algae, particularly marine algae, received much attention due to their ability to uptake and accumulate of heavy metals, and also make them ideal candidates for the selective removal and absorption of heavy metals.
According to (Deng et al. 2013; Smrchek & Zeeman 1998; Mohan & Hosetti 1999) the results indicate that diatoms’ cell wall is an importance protective shield against mercury; when mercury enters the cell, it changed to other chemical forms by reacting with sulfur proteins as glutathione, which reduces the toxicity.
In spite the fact that toxicity tests with isolated species can provide useful indications for environmental risk assessment of the test compound, they cannot predict changes in a natural community at different organizational levels (Bérard et al. 2003).
Assessment of impacts of chemical contamination on the environment should account for the natural variability of biological systems in space and time, particularly any endpoint used to evaluate toxicity may be expected to vary in magnitude under different environmental and biological factors (Schindler 1987).
According to Kaoutar & Mourad (2013), Phytochelatins are glutathione oligomers produced by Phytochelatins syntheses. They are present at plants, fungus, nematode, algae and diatoms. Phytochelatins are acting as chelators and are important for heavy metals detoxification (Saadatmand & Niazi 2015). The mercury interactions with other elements will be affected by the biotransformation of HgII with microorganisms, also there are many studies and information on the genetics and biochemistry of Hg (Baldi et al. 1993; Essa et al. 2002; King et al. 2000; Silver & Misra 1988; Silver & Walderhaug, 1992), and however, the quantitative data are rare and needed to pay attention. The reduction of Hg to Hg0 (Frischmuth et al. 1993; Morel et al. 1998), and its methylation (Hamdy & Noyes 1975) are important; however, it is necessary to focus on the mercury biogeochemistry (Amyot et al. 1994). According to Anantharaj et al. (2011) the intracellular HgCL2 changes to Hg -CH3, in some species of diatoms (for example, Amphora coffeformis (Agardh) Kütz. Nitzschia, and also forms metal/H+ antiporter complex, which is transferred to vacuole through one of the tonoplast present antiporters (Kaoutar & Mourad 2013). The metal complexes become more stable by attaching to sulfide ions (Ruley et al. 2005). Damage caused by heavy metals are dependent to free radicals, types of oxygen reactions and removal mechanisms by plants. As claimed by some researchers, oxidative damage by heavy metals are result of fat peroxidation or nucleic acid and proteins oxidation, chlorophyll destruction and photosynthesis impair.
According to Kaoutar & Mourad (2013), diatoms accumulate heavy metals as results of tolerance mechanisms. They synthesize active ligands such as phytochelatins, metallothioneins, amino acids and organic acids that can form complexes with heavy metals and translocate them into vacuoles.
This investigation was focused on the ability of Nitzschia capitellata in accumulation of heavy metal, Together with investigating the concentration of the metals absorbed by N. capitellata.
Materials and Methods
F/2 medium preparation
The F/2 medium was used for diatoms’ culture (Andersen et al. 2005). The compounds of Tab. 1. were added to 1950 ml filtered river water and the final volume was increased with deionized water to two liters (Tab. 1.).
| Dd | Stock | Component | |
| 1 mL | 15 | NaNO3 | |
| 1 mL | 1 | NaH2PO4·H2O | |
| 1 mL | 3 | Na2SiO3·9H2O | |
| 1 mL | see recipe | Trace metals stock | |
| 0.5 mL | see recipe | Vitamins stock | |
| Element Stock | |||
| Add | Stock g in 100 ml (dH2O) | Component | |
| 0.88 g | - | Na2EDTA·2H2O | |
| 0.63 g | - | FeCl3·6H2O | |
| 0.2 mL | 1.79 g | MnCl2·4H2O | |
| 0.2 mL | 0.219 g | ZnSO4·7H2O | |
| 0.2 mL | 0.099 g | CoCl2·6H2O | |
| 0.2 mL | 0.098 g | CuSO4·5H2O | |
| 0.2 mL | 0.062 g | Na2MoO4·2H2O | |
| Vitamins Stock | |||
| 0.04 g | - | Thiamine · HCl (vitamin B1) | |
| 1 mL | 0.02 in 100 | Biotin (vitamin H) | |
| 0.2 mL | 0.01 in 10 | Cyanocobalamin (vitamin B12) | |
Table 1. F/2 Medium.
Diatom purification
The light microscope (model Olympus CX31) was used for observation of morphological characteristics of diatoms. In order to identify diatom species, we used the following website: http://westerndiatoms.colorado.edu.
The diatom was isolated by serial dilution method and transferred to solid medium (agar) and kept at 17 ± 21°C and 16-8 period light under 2500 lux. The colonies of diatoms were formed after 20 days, then they were investigated with light microscope and transferred to liquid medium (F/2) to increase biomass. The liquid medium was shacked manually (daily) in order to increase growth and biomass. The MOPS (3-N morpholino propane sulfonic acid) was used to adjust pH of medium culture (Deng et al. 2013). All the steps of culturing were done under sterile conditions.
Solution and reagents
100 mg/l mercury solution stock was provided (HgCL2 0.013 gr amount was solved in 70 ml distilled water then, 1 ml HNO3 concentration was added to it and the final volume adjusted to 100 ml). 1-5 μg/l mercury standard solutions were provided from mercury solution stock using distilled water that contained 10 ml concentration acid nitric (daily). This concentration was used to provide 10 and 1 mg/l of HgCl2 (1000 μg/l), then it was used to make 90, 285 and 300 μg/l in 125 ml concentration volumes. 100 ml of the mercury standard solution and 100 ml of controlled distilled water were added to flasks separately. 5 ml concentrated sulfuric acid and 2.5 ml nitric acid was added to each flask, then 15 ml potassium permanganate solution was added to them. After 15 minutes, 8 ml potassium permanganate solution was added to each then it was warmed in the 95°C water bath for 2 hours. The sufficient amount of NaCl-hydroxylamin solution was added to remove extra potassium permanganate. 25 gr of SnCL2 was solved in distilled water that was containing 20 ml HCL concentration; then, final volume was adjusted to 100 ml with distilled water. SnCL2 solution used for reduction of Hg2+ to Hg. The signals were recorded by apparatus when mercury vapor was moving into absorbent cell. At first, the system was washed with distilled water and then standard was placed in same situation. Standard Curve was drawn by mercury micrograms maximum points. The atomic absorption spectrometer was used to analysis of samples. It was set at 253.7 nm wavelengths for mercury, and then calibration curve produced. In the next step, the amount of mercury in the treatment samples was measured via calibration curve (Fig. 1.) (Eaton et al. 2005).
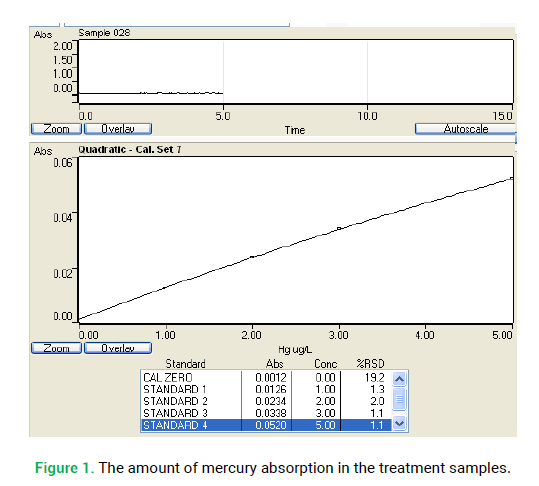
Figure 1: The amount of mercury absorption in the treatment samples.
Before adding HgCl2, the 0.01 mole of MOPS was provided to set the pH of culture medium at 7.4 (Deng et al. 2013). The dithioerythreitol (DTE) 0.01 mole L-1 solution was provided for mercury separation that bonded to the cell wall (Deng et al. 2013; Kelly et al. 2006). (0.3856 gr DTE amount was solved in 250 ml distilled water). DTE has two sulfide bands that can react with mercury to produce Hg-S (Deng et al. 2013).
Atomic absorption spectroscopy method
30 ml of the culture medium was centrifuged at 3500 rpm for 4 minutes, then the cells from 1ml of sediment was counted using Sedgewick Rafter slide. The absorption of sediment (diatom) and culture medium without treat, with no mercury was measured via atomic absorption Spectrometer. A triplicate of each concentration (90, 285, 300 μg/l) of HgCl2 and control was provided. 30 ml culture medium of diatoms was centrifuged and 1ml of concentrated diatom with 350 × 103 cell/Cm2 cell density was added to each mercury concentration. The treatment solution was centrifuged and absorption recorded via Atomic Absorption Spectrometer with considering the dilution factor of solution. The cells were centrifuged after 30 minutes treatment, and then the mercury absorbance was measured. To release cell wall attached to mercury, the sediment (diatoms) was mixed in 10 ml of DTE 0.01 mole L-1 and centrifuged after 10 minutes, at 2500 rpm for five minutes (Kelly et al. 2006). The pellet was washed with DTE again and the absorbance was detected by considering the dilution factor. Thus, the mercury attached to the cell walls was calculated. The diatoms without mercury attached to cell wall were digested with 1ml concentrated HCL and 1ml concentrated HNO3. It was placed in water bath for an hour, then the absorption was detected by considering dilution and finally the intracellular mercury amount was calculated.
Statistical analysis
Data were analyzed using ANOVA (completely randomized) to determine if there were significant differences among the obtained means. T-tests were carried out to determine if there were significant differences (p ≤ 0.05 and p ≤ 0.1) between individual treatments (SPSS-13).
Results and Discussion
The pure colonies of diatoms were transferred to liquid medium to increase the biomass (Fig. 2a-2d).
Figure 2: The pure colonies of Nitzschia capitellata Hustedt (a, b, c and d).
The most important target of this study was to survey the surveillance of diatoms in presence of heavy metal and resistance of cells to high concentrations of mercury. For this purpose, initially, the diatoms were exposed into 1000 μg/l concentration of HgCl2. The results of our study showed that diatoms died after five days when they were suddenly exposed to 1000 μg/l concentration of HgCl2 (Fig. 3.), but when those diatoms were gradually exposed at tolerable mercury concentrations, they were able to survive and adapted themselves to toxicity conditions.
Figure 3: a: Before treatment; b: Next treatment after five days, all of the cells were destroyed at 1000 μg/l concentration of mercury.
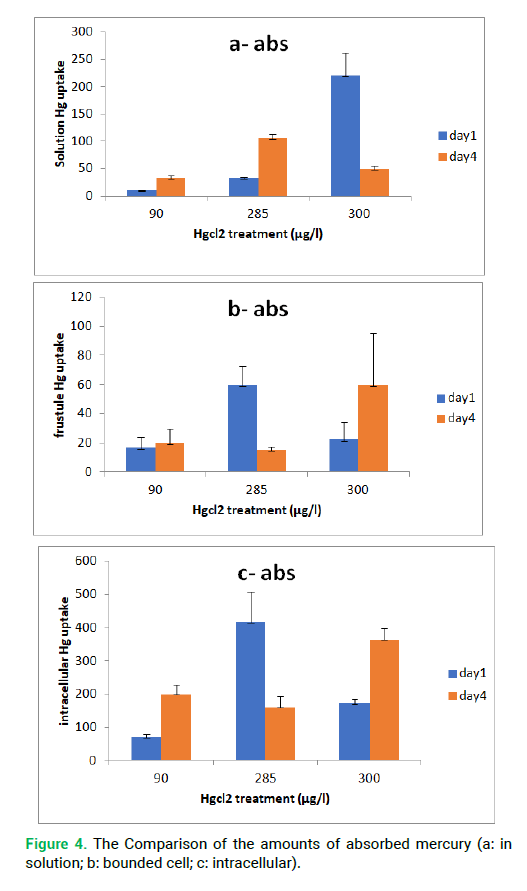
The measurement was done for three concentrations for the first and fourth days. The amounts of mercury absorption in the solution, cell wall and intracellular have been shown in Fig. 4.
Figure 4: The Comparison of the amounts of absorbed mercury (a: in solution; b: bounded cell; c: intracellular).
The absorption amounts were measured in first and fourth days. The diatoms were able to accumulate 450 μg/l of HgCl2 during the first day; however, after providing detoxification defensive conditions the diatoms were able to discharge over 250 μg/l mercury to out of cells. The mean of the remained mercury in solution was 10.16 μg/l, (in first day for 90 μg/l concentration). On the other hand, the amount of the absorbed mercury was 79.84 μg/l. Diatoms were able to accumulate 70.33 μg/l mercury in intracellular (based on intracellular comparison). The mercury concentration was 34 μg/l in solution in the fourth days, and the amount of the absorbed mercury was 56 μg/l. The intercellular mercury absorption was 199.5 μg/l at the fourth days, which means that after four days the diatoms were able to accumulate 129.17 μg /l of mercury without discharging to outside cells. The amount of absorption by cell wall increased from 16 to 19 μg/l comparing the fourth day with the first day, which is not very high (for 90 μg/l concentration). Comparing the fourth and first days, the amount of mercury in solution decreased from 79.8 to 57.67 μg/l. On the other hand, 22.2 μg/l of the mercury decreased, whereas the intracellular concentration increased. The mean of the remaining mercury in solution was 32 and 106 μg/l, in other words, the amount of the absorption mercury was 252.59 and 179 μg/ l, respectively for first and fourth days (in the 285 μg/l concentration). The amount of intercellular mercury absorption was 416.5 and 160 μg/l respectively in the first and fourth days (for concentration 285 μg/l). In other words, after four days the diatoms were able to discharge 256.5 μg/l of mercury to environment or cell wall. The amount of absorbed mercury decreased from 252.59 to 179 μg/l in fourth day in 285 μg/l concentration. On the other hand, 73.59 μg/l of absorbed mercury decreased. The Amount of intracellular mercury decreased and discharged to solution, which indicated the toxicity for diatoms. The mean of the remaining mercury in solution was 220 and 251.1 μg/l, and the intercellular absorption of mercury was 171.33 and 363.66 μg/l. The mean for cell wall in 300 μg/l concentration was 22.18 and 59.75 μg/l, respectively for first and fourth days. The intracellular, cell wall and solution concentrations increased after four day in the 300 μg/l concentration. The amount of absorption in solution increased from 80 to 251.1 μg/l, which confirmed intercellular accumulation of metal. On the other hand, the amount of cell wall absorption increased 37.57 μg/l comparing with the first day. In this condition, the cell wall acts as a protective shield. The intracellular concentration became 450 μg/l (at intracellular corporation) and discharged to the outside of the cell (for 250 concentration), but the discharge for intracellular concentration (in 300 μg/l) was less than 400 μg/l and could not create toxicity for diatoms or it made de toxicity factors. The obtained results in this study and the mean of mercury bonded to the cell wall in 90 and 300 μg/l at the first and fourth days were in line with the results of Phaeodactylum tricornutum, in which the intracellular concentration increased from 126 μg/l to 2229 μg/l (Deng et al. 2013). However, the amount of mercury decreased in 285 μg/l at fourth day. Probably the reason of metal concentration fluctuation in cell wall was due to intracellular condition and detoxification mechanisms.
The diatoms survived when they were exposed to less concentrations of HgCl2 and produced the necessary detoxification factors. The T-test analysis was significant at the level of 0.01 between first and four days comparing for the remaining in solutions and intracellular mercury for all concentrations, but it was significant at the level of 0.05 for bonded cell wall mercury in HgCl2 concentration of 285 μg/L.
Conclusion
According to studies, the cell wall of diatoms is able to prevent the entering mercury to intracellular at shocking concentrations. The diatoms may send signals to the cell wall or may have receptors in cell wall surface to connect to metal ions. As a result, the cell wall plays an important role in the entrance or discharge of the metals, which is related to intracellular concentration and metal toxicity. Probably the diatoms have defense mechanism for hard conditions, but they may be destroyed when suddenly contact with shocking conditions. They do not have enough time to create detoxification factors at shocking conditions as happened for the Nitzschia capitellata in 1000 μg/l mercury concentration. There are many studies on the use of heavy metals for microorganisms’ treatment such as diatoms and cyanobacteria. A few of them were about mercury metal due to high toxicity and sensitive safety considerations. The Cold-Vapor Atomic Spectrometry (CVAS) method has been used by many researchers to analyze the mercury amount in the organisms needed to reduce Hg to Hg0. According to studies, the total ionic mercury can be measured by the acid reduction method while the organic and inorganic mercury can be distinguished through alkaline method by adding CdCl2 to separate the C-Hg in samples.
Depending on studies on diatoms treatment have been done with heavy metals such as Cadmium, Copper, chrome, Nickel and Arsenic in over 1000 μg/l concentration. Also, the study for Nitzschia sp. treat has been done with different concentrations of copper and cadmium until 16 ppm treatment, in which the diatoms showed high resistance at that concentration. In the coastal waters of some Asian countries, the amount of 100 to 120 μg/l of mercury concentration has been reported.
Along with a general reduction in community biomass, there are several reports that also demonstrate some associated structural changes to the communities. It has been suggested that changes occurring in species composition of periphyton communities experiencing heavy metal contamination are due to selection of those species that are tolerant to the pollution. However, in some studies they provide adequate evidence indicating that the structural changes observed in metal-exposed community have actually resulted from direct selection against sensitive species. Several researchers have considered that there are strong interactions between different components of biofilms, such as bacteria, microalgae and ciliates, meaning that establishment of causal relationships are difficult. Part of the resilience of microbenthic communities to metal stress may be due to the input of organic waste providing material for the sorption and chelation of metal ions.
European Union has been allowed to discharge 50 μg/l of mercury at surface waters. The 120 μg/l amount of mercury prevented the growth of Phaeodactylum tricornutum. In general, the Hg concentration in the sea water is lower than 0.12 μg/l, although, the Hg concentration in some polluted areas is as high as 2.3 μgL-1 or even 260 μg/L. At first, the diatom was exposed at 1000 μg/l concentration of HgCL2 to recognize its capability to tolerate mercury amount without previous preparation. It is predicted that probably the diatom would die without creating a defensive factor. The results showed that all of the cells were destroyed at 1000 μg/l concentration of mercury after five days, so we used the lower concentrations of HgCl2. Probably the detoxification of diatoms will not be perfect when they do not have enough time to create defense mechanisms.
The microorganisms and other creatures will die in shocking mercury concentrations. The diatoms should be gradually exposed to HgCL2 concentrations (from low to high amounts). They are able to adapt to the new condition or they cannot tolerate the new situation. According to the other studies we selected 90 μg/l concentration of HgCl2, as standard of surface waters and some waters of the coastal area of Asia. According to the fact that, diatoms are able to remove mercury pollution from environment and also the most important goals of this study to select the tolerant and lethal concentrations of mercury, it is useful to use diatoms in industrial environments to help accumulate mercury and make safety effluent (Hg2+ is converted to Hg) by diatoms. The 285 and 300 μg/l concentrations of HgCl2 were anticipated for shocking concentrations because diatoms died at 1000 μg/l concentration mercury. Therefore, the survival of diatoms was more important than growth rates. We used DTE 0.01 mole L-1 for separation of mercury bonded to cell wall and calculated intracellular mercury. The intracellular mercury reflects diatoms’ compatibility with metals. The DTE has two sulfide bands, is able to react with mercury and produces S-Hg. The cell wall bond metals may enter into environment or cell. Releasing of mercury from the cell into the environmental represents the mechanism of detoxification by the diatoms. We have to mention that intracellular increasing concentration of mercury in comparison with solution concentration is different (the intracellular volume of the solution volume is much smaller). The diatoms are able to convert mercury to other mercury forms (R-Hg., for example CH3-Hg). The intracellular increasing of mercury happens based on pre-concentration principle and is accumulated during a period. Discharging the mercury to out of cell by diatoms demonstrates the preparation and act of detoxifying mechanism. The intracellular mercury is very lower than extracellular (solutions’ volume), and they must not be compared with each other. We do not have to expect the equality of the aggregation of intracellular and cell wall mercury concentrations with the mercury concentration in the solution, because of the very small volume of intracellular space for mercury accumulating.
Acknowledgements
The authors would like to appreciate Dr. Farhad Mosakhani, manager of Mabna laboratory for his valuable help in study.
References
Jain C.K. 2004. Metal fractionation study on bed sediments of river yamuna India. Water Res. 38: 569-578. https://doi.org/10.1016/j.watres.2003.10.042
Saadatmand S., Niazi A. 2015. Investigating the purification of contaminated water supplies by heavy metals such as cupper and cadmium using diatom algae. J Agric Sci. 7: 5-18. http://dx.doi.org/10.5539/jas.v7n5p5
Leung C.M., Jiao J.J. 2006. Heavy metal and trace element distributions in groundwater in natural slopes and highly urbanized spaces in mid-levels area, Hong Kong. Water Res. 40: 753-767. https://doi.org/10.1016/j.watres.2005.12.016
Deng G., Zhang T., Yang L., Wang Q. 2013. Studies of bio uptake and transformation of mercury by a typical unicellular diatom Phaeodactylum tricornutum. Chin Sci Bull. 58: 256-265. https://doi.org/10.1007/s11434-012-5514-3
Wilde E.W., Benemann J.R. 1993. Bio removal of heavy metals by the use of microalgae. Biotechnol Adv. 11: 781-812. https://doi.org/10.1016/0734-9750(93)90003-6
Smrchek J.C., Zeeman M. 1998. Assessing risks to ecological systems from chemicals. Chapter 3 In: Handbook for environmental risk assessment and management, (ed.), P. Calow. 24-90. Blackwell Science. Ltd., London.
Mohan B.S., Hosetti B.B. 1999. Aquatic plants for toxicity assessment. Environ Res. 81: 259-274. https://doi.org/10.1006/enrs.1999.3960
Bérard A., Dorigo U., Mercier I., Becker-van Slooten K., Grandlean D., Leboulonger C. 2003. Comparison of the eco toxicological impact of the traizines Irgarol 1051 and atrazine on micro algal cultures and natural micro algal communities in Lake Geneva. Chemosphere. 53: 935-944. https://doi.org/10.1016/S0045-6535(03)00674-X
Schindler D.W. 1987. detecting ecosystem responses to anthropogenic stress. Can J Fish Aquat Sci. 44: 6-25. https://doi.org/10.1139/f87-276
Kaoutar B.C., Mourad B. 2013. The role of algae in phytoremediation of heavy metals: A review. J Mater Environ Sci. 4: 873-880.
Baldi F., Pepi M., Filippelli M. 1993. Methylmercury resistance in Desulovibrio desulfuricans strains in relation to methylmercury degradation. Appl Environ Microbiol. 59: 2479-2485.
Essa A.M.M., Macaskie L.E., Brown N.L. 2002. Mechanisms of mercury bioremediation. Biochem Soc Trans. 30: 672-674. https://doi.org/10.1042/bst0300672
King J.K., Kostka J.E., Frischer M.E., Saunders F.M. 2000. Sulfate reducing bacteria methylate mercury at variable rates in pure culture and in marine sediments. Appl Environ Microbiol. 66: 2430-2437. https://doi.org/10.1128/aem.66.6.2430-2437.2000
Silver S., Misra T.K. 1988. Plasmid-mediated heavy metal resistances. Annu Rev Microbiol. 42: 717-743. https://doi.org/10.1146/annurev.mi.42.100188.003441
Silver S., Walderhaug M. 1992. Gene regulation of plasmid- and chromosomal-Determined inorganic ion transport in bacteria. Microbiol Rev. 56: 195-228. https://doi.org/10.1128/MMBR.56.1.195-228.1992
Frischmuth A., Weppen P., Deckwer W.D. 1993. Microbial transformation of mercury (II). I. Isolation of microbes and characterization of their transformation capabilities. J Biotechnol. 29: 39-55. https://dx.doi.org/10.1128/aem.01794-60
Morel F.M.M., Kraepiel A.M.L., Amyot M. 1998. The chemical cycle and bioaccumulation of mercury. Annu Rev Ecol Syst. 29: 543-566. https://doi.org/10.1146/annurev.ecolsys.29.1.543
Hamdy M.K., Noyes O.R. 1975. Formation of methylmercury by bacteria. Appl Microbial. 30: 424-432.
Amyot M., Mierie G., Lean D.R.S., McQueen D.J. 1994. Sunlight induced formation of dissolved gaseous mercury in lake waters. Environ Sci Technol. 28: 2366-2371. https://doi.org/10.1021/es00062a022
Anantharaj K., Govindasamy C., Natanamurugaraj G., Jeyachandran S. 2011. Effect of heavy metals on marine diatom Amphora coffeaeformis (Agardh. Kutz). Glob J Environ Res. 5:112-117.
Ruley A., Sharma N., Sahi Sh. 2005. Antioxidant defense in a lead accumulating plant, sesbania drummondii. Int J Plant Physiol Biochem. 42: 899-906. https://doi.org/10.1016/j.plaphy.2004.12.001
Andersen R., Berges J., Harrison P., Watanabe M.M. 2005. Appendix A-recipes for freshwater and seawater media. Algal Culture Techniq. https://doi.org/10.1016/b978-012088426-1/50027-5
Eaton A.D., Clesceri L.S., Greenberg A.E., Franson. M.A.H. 2005. Standard methods for the examination of water and wastewater. Am J Public Health. https://trove.nla.gov.au/work/16646325
Kelly D., Budd K., Lefebvre D.D. 2006. Mercury analysis of acid- and alkaline- reduced biological samples: Identification of meta-cinnabar as the major bio transformed compound in algae. Appl Environ Microbiol. 72: 361-367. https://doi.org/10.1128/aem.72.1.361-367.2006
Baselt R.C. 1980. Biological monitoring methods for industrial chemicals. Biomedical Publications, Davis, Calif. https://doi.org/10.1093/clinchem/27.3.516
Daniels R.S., Wigfield D.C. 1989. Cold-vapor mercury atomic absorption spectrometry. Sci Total Environ. 8: 319-323. https://doi.org/10.1016/0048-9697(89)90273-8
Daniels R.S., Wigfield D.C. 1989. Cold-vapor mercury atomic absorption spectrometry II. Acidic versus alkaline reduction. Sci Total Environ. 89: 325-329. https://doi.org/10.1016/0048-9697(89)90274-X
Daniels R.S., Wigfield D.C. 1993. Cold-vapor mercury atomic absorption spectrometry: HCl as the cause of the double peak phenomenon. J Anal Toxicol. 17: 196-198. https://doi.org/10.1093/jat/17.4.196
Falandysz J., Chwir A. 1997. The concentrations and bio concentration factors of mercury in mushrooms from the Mierzeja Wislana sand-bar, Northern Poland. Sci Total Environ. 15: 221-228. https://doi.org/10.1016/S0048-9697(97)00150-2
Gill U., Bigras L., Schwartz H. 2004. Routine, automated determination of inorganic and total mercury in multimedia using cold vapor atomic absorption spectrometry. Chemosphere. 56:1097-1103. https://doi.org/10.1016/j.chemosphere.2004.05.014
Locatelli C., Torsi G. 2001. Heavy metal determination in aquatic species for food purposes. Ann Chim. 91: 65-72.
Hatch W.R., Ott W.L. 1968. Determination of sub-microgram quantities of mercury by Atomic absorption spectrophotometry. Anal Chem. 40: 2085-2087. https://doi.org/10.1021/ac50158a025
Magos L. 1971. Selective atomic-absorption determination of inorganic mercury and Methylmercury in undigested biological samples. Analyst. 96: 847-853. https://doi.org/10.1039/AN9719600847
Panda K.K., Lenka M., Panda B.B. 1992. Monitoring and assessment of mercury Pollution in the vicinity of a chlor alkal plant. III. Concentration and genotoxicity of mercury in the industrial effluent and contaminated water of Rushikulya estuary, India. Mutat Res. 280: 149-160. https://doi.org/10.1016/0165-1218(92)90043-Y
Wetzel R.G., Likens G.E. 1991. Limnological analyses, 2nd ed. Springer-Verlag, New York, N.Y.
Mance G. 1987. Pollution threat of heavy metals in aquatic environments. Elsevier Applied Science, New York, N.Y.
Fitzgerald W.F., Lamborg C.H., Hammer Schmidt C.R. 2007. Marine biogeochemical Cycling of mercury. Chem Rev. 107:641-662.
Feng X.B., Dai Q.Q., Qiu G., Li G., He L., Wang D. 2006. Gold mining related mercury contamination in Tong guan, Shaanxi Province, PR China. Appl Geochem. 21: 1955-1968. http://dx.doi.org/10.1016/j.apgeochem.2006.08.014
Li P., Feng X.B., Shang L.H., Qiu G.L., Menu B., Liang P., Zhang H. 2008. Mercury pollution from artisanal mercury mining in Tongren, Guizhou, China. J Appl Geochem. 23: 2055-2064. http://dx.doi.org/10.1016/j.apgeochem.2008.04.020