Research - Modern Phytomorphology ( 2022) Volume 16, Issue 2
Salicylic acid-induced amelioration of salt stress by modulating morpho-biochemical attributes and antioxidant mechanism in black gram (Vigna mungo L.)
Arsheed Ahmad Rather1*, Sabanayagam Natarajan1, Ali Raza2*, Sidra Charagh3 and Talha Javed42College of Agriculture, Oil Crops Research Institute, Fujian Agriculture and Forestry University (FAFU), Fuzhou, China
33State Key Laboratory of Rice Biology, China National RiceResearch Institute, Chinese Academy of Agricultural Sciences (CAAS), Hangzhou,, China
4College of Agriculture, Fujian Agriculture and Forestry University (FAFU), Fuzhou, China
Arsheed Ahmad Rather, Department of Botany, Annamalai University Tamil Nadu, India, Email: arshidrather199@gmail.com Ali Raza, College of Agriculture, Oil Crops Research Institute, Fujian Agriculture and Forestry University (FAFU), Fuzhou, China, Email: alirazamughal143@gmail.com
Received: 25-Apr-2022, Manuscript No. mp-22-61752; Accepted: 25-May-2022, Pre QC No. mp-22-61752 (PQ) ; Editor assigned: 26-Apr-2022, Pre QC No. mp-22-61752 (PQ) ; Reviewed: 23-May-2022, QC No. mp-22-61752 (Q) ; Revised: 24-May-2022, Manuscript No. mp-22-61752 (R) ; Published: 02-Jun-2022, DOI: 10.5281/zenodo.7735570
Abstract
As a severe and prevalent abiotic stress, salinity causes extensive crop losses by limiting plant growth and production worldwide. The present study was carried out to mitigate the salinity-induced harmful effects on plant growth, biochemical and antioxidant attributes of black gram by the foliar application of Salicylic Acid (SA). Vigna mungo L. Hepper (ADT-5) plants were grown in salt-treated (75 mM NaCl) and untreated (0 mM NaCl) growth medium. Three levels of SA (0.5 mM, 0.75 mM, 1.00 mM) were applied through the foliar spray. Salt stress significantly reduced the morpho-biochemical attributes in black gram. However, foliar application of 0.5 mM SA displayed ameliorated response under salt stress, as evidenced by the highest values for growth attributes and photosynthetic pigments compared to control and other experimental units. A reduced photosynthetic pigment (chlorophyll a, b, and carotenoids) under salt stress was significantly ameliorated by foliar application of 0.5 mM SA. The proline and enzymatic antioxidants like catalase, and superoxide dismutase activities were improved by salicylic acid application compared to the control group. This study indicated that SA reduced the deleterious effect of salt stress under high salt concentration by regulating morphological and biochemical indices in black gram.
Keywords
Salinity stress, salicylic acid, proline, malondialdehyde, hydrogen peroxide, antioxidant enzymes
Introduction
Agricultural productivity around the globe is under severe threat due to various biotic and abiotic stressors. Salt stress is the most prevalent abiotic threat the world faces in arid and semi-arid areas. The soluble salt concentration in these areas is high, and the rainfall is low, which allows the percolation of salts (Zhoa et al., 2007; Saddiq et al., 2021; Sharma et al. 2022). Salt stress reduces plant growth by osmotic and ionic effects that disturb ion homeostasis (Athar and Ashraf 2009; Zafar et al., 2017). Salt stress disrupts all morphological, physiological, anatomical, and biochemical parameters of a plant (Iqbal et al., 2018).The growth and development of plants, as affected by salt stress, cause a threat to global food production. It affects plant physiology by damaging cellular organelles, inhibiting enzyme activities and protein synthesis, cessation of photosynthesis and respiration, changing nutrient uptake, reducing soil osmotic potential, as well as hindering water uptake by roots (Talaat 2019; Hasanuzzaman et al., 2020). Continuous accumulation of Na+ and Cl- leads to overproduction of Reactive Oxygen Species (ROS) like singlet oxygen (1O2), hydrogen peroxide (H2O2), hydroxyl radicles (.OH), and superoxide ions (O2 -) in plant cell organelles (Hasanuzzaman et al., 2020). Higher and non-metabolized ROS leads to oxidative stress, affecting cellular metabolism, cell division, growth, membrane integrity, and photosynthetic function (Nazar et al., 2015). To restain ROS accrued upshot, plants upregulate enzymatic and non-enzymatic antioxidant defence systems to scavenge them. Salinity also hinders the uptake of certain essential elements like K+ and negatively impacts plant water relations (Deinlein et al., 2014). The overall reduction in growth and development of plants under salt stress is the cumulative effect of disruption in ion homeostasis, water balance, and disruption in photosynthetic capacity (Khan et al., 2012).
Black gram [Vigna mungo (L.) Hepper] occupies an important place among the premier pulse crops in India. Black gram is an extensively grown grain legume and belongs to the Fabaceae family and got noticeable significance from the point of food and nutritional security in the world (Thakur et al., 2017). Black gram is a perfect combination of all nutrients, including 20% to 25% proteins, 40% to 47% starch, ash fats, carbohydrates, and essential vitamins (Manjri et al., 2018). India produces 1.45 million tons of black grams annually on about 3.25 million hectares of land (Arulbalachandran et al., 2010).
The approach and tactics to mitigate salt stress’s adverse effects on plants have been an important in scientific research (Raza A. 2022). Improvement in stress tolerance in plants has significant implications for agriculture and horticulture (Rajeshwari and Bhuvaneshwari 2017). Plants growing in the saline environment have evolved diverse adaptive strategies to overcome salt stress at both cellular and whole plant levels. The osmotic stress is compensated by accumulating proline, amino acids, carbohydrates, and proteins (Ahmad et al., 2017). Several physiological and genetic manipulations have been in higher plants, which results in Na+ exclusion at root surface and decreases root to shoot translocation of Na+ (Schubert et al., 2009).
Phytohormones are essential regulators for the growth and development of plants (Sabagh et al., 2021). They play a vital role in regulating the response of plants to different biotic and abiotic stresses. Salicylic acid (SA., 2-hydroxy benzoic acid), a phenolic endogenous plant growth regulator, acts as a signaling molecule for modifying plant response by inducing the expression of genes that code defense-related compounds like jasmonic acid and proline (Wani et al., 2017; Shaukat et al. 2022). It acts as a bio stimulator that activates the biochemical pathways associated with salt tolerance mechanisms in plants (Fariduddin et al., 2018). Salicylic acid alters plant response to abiotic stressors by signaling cross-talk with other plant hormones (Iqbal et al., 2015). Salicylic acid plays a vital role in assimilating nutrients under stressful conditions (Khan et al., 2015).
Salicylic acid is the strong candidate among stress ameliorators and has been recognized as plant growth premotor. It plays a diverse role in plants, including stomatal closure, thermogenesis, flower induction, and abiotic stress resistance. Eraslan (2007) reported that foliar spray of SA enhances plant growth, photosynthetic pigment, and antioxidant activity of carrot root and shoot. Foliar spray of SA has also been reported to maintain K+/Na+ ratio and boost photosynthetic activity and antioxidant system under stressful conditions (Hayat and Ali. 2010). Black gram is a very sensitive crop to high salt concentrations like many other legumes (Muas and HofSman, 1977). Several studies have shown that the application of SA improved plant salt tolerance by acting as chemical messengers in numerous metabolic processes such as mineral nutrition uptake, antioxidant activity, methylglyoxal detoxification, photosynthesis, respiration, cellular signaling, senescence, and overall cellular redox homeostasis (Bukhat et al., 2020; Hoang et al., 2020; Kaya et al., 2020; Es-sbihi et al. 2021; Shamili et al., 2021).
There is also little information available in the literature on optimal concentrations of SA required for achieving maximal productivity on salt-affected soils. In this study, the effects of SA were examined on black gram under non-saline and saline conditions. The current experiment was designed to evaluate the varied SA concentrations as a foliar application on growth, biochemical and antioxidant profile in the leaves of black gram plants and the possibility to ameliorate salt stress.
The optimal levels of SA are species-or even cultivardependent. Furthermore, previous studies focus on the individual effects of SA improving crop plant tolerance, while their putative integrative effects have rarely been addressed. The study was conducted to find out the (1) impact of salt stress on several morpho-biochemical, and antioxidant activities of the subject plants. (2) To analyze the response of black gram genotype towards exogenously applied SA concentration with reference to these parameters under salt stress. The information obtained from this experiment will give a future direction for expanding this crop under saline soil. We hypothesized that the foliar-applied SA alone or in combination with salt stress might reduce the detrimental effect of salinity stress by modulating growth, biochemical parameters, and increasing antioxidant activity in the black gram plant.
Materials and Methods
Plant material and stress treatment
The present study was carried out at the Department of Botany, Annamalai University, Annamalai Nagar (Lat 110 24’N and Long 790 44’ East) with Black gram genotype variety ADT-5 under salt stress. Healthy seeds of black gram genotype variety ADT-5 were procured from Pulse Research Institute, Pudukottai Tamil Nadu, India. The surface sterilization was done by using Mercuric chloride (0.01%) followed by washing with double distilled water. The seeds were sown in pots containing a mixture of soil, sand, and manure in the ratio of 1:2:1. The pots were arranged in Completely Randomized Block Design (CBRD). Ten-day-old seedlings were subjected to 0 mM NaCl and 75 mM NaCl (to facilitate the seedling establishment stage) given in three successive doses on alternate days to avoid osmotic shock. Treatment was continued till the required concentration of salt was achieved. The experimental soil was sandy loam with 7.4 pH and EC values of 0.4 dSm-1 and 8.3 dSm-1 were achieved in soils, which were maintained throughout the experimental trials by monitoring at weekly intervals. After six days of emergence, thinning was done, and three plants were maintained per pot throughout the experiment.
Foliar application of salicylic acid
Salicylic Acid (SA) was obtained from Himedia chemical laboratory Chennai (RM 1476-500G). The hormonal solution was prepared by dissolving it in 5 mL ethanol, and desired concentration was achieved by the addition of distilled water. After that, surfactant teepol (0.5%) was added with SA treatment solution. SA solution of different concentrations (0.5 mM, 0.75 mM, and 1.00 mM) were sprayed 20 days after sowing with the help of a hand sprayer. One group of plants was sprayed with distilled water and treated as a control.
Research design
The experimental design consisted of one salt concentration of 75 mM NaCl combined with three SA concentrations of 0.5 mM, 0.75 mM, and 1.00 mM. One group of plants was treated as a control and was not subjected to salinity stress (T0) or SA spray. Other treatment groups consisted of 75 mM NaCl (T1), 0.5 mM SA (T2), 0.75 mM SA (T3), and 1.00 mM SA (T4), 75 mM NaCl plus 0.5 mM SA (T5), 75 mM NaCl plus 0.75 mM SA (T6), and 75 mM NaCl plus 1.00 mM SA (T7). The pots were arranged in Completely Randomized Block Design (CBRD). After 35 days, plant morphology, biochemical and antioxidant assays were carried out.
Analysis of growth and biomass
After completion of the experiment (35 days), five plants were randomly selected from each treatment. The root length and shoot length were measured by using a meter scale and were expressed as (cm plant-1). After careful washing and drying, the plant parts were separated and oven-dried at 80°C. Dry weight was measured to determine the accumulation and allocation of biomass and was expressed in (grams).
Biochemical analysis
Photosynthetic pigment: To extract the photosynthetic pigment, 0.5 g of fresh leaves were grounded in a porcelain mortar with 15 ml of acetone 80% and then centrifuged for 10 minutes at 4000 rpm. Absorption of the extract was read by UV/Vis spectrophotometer at 645 mm, 663 mm, and 480 nm wavelengths for chlorophyll a, b, and carotenoid. For device adjustment, acetone 80% was used. Chlorophylls contents per gram of fresh weight were calculated (Arnon’s method, 1949).
Proline assay: Proline accumulation level in the leaf samples was determined based on proline’s reaction with ninhydrin following the method of Bates et al. (1973). Briefly, 0.5 g of leaf sample from each treatment was macerated with 10 mL of 3% sulphosalicylic acid, and the homogenate was filtered through filter paper. The mixture was heated at 100°C for 1 hr. in a water bath after the addition of 2 mL of 1% ninhydrin, and 2 ml of 75% glacial acetic acid. The reaction was arrested in an ice bath, and the chromophore was extracted with 4 ml toluene, and its absorbance at 520 nm was determined through spectrophotometer.
Total Protein Content: Total protein content was estimated using the Lowry et al., (1951) method. Fresh tissue weighing 0.5 g was macerated in 20 percent Trichloroacetic acid using mortar and pestle. The homogenate was centrifuged at 600 rpm for 30 minutes, and the supernatant was discarded. Five ml of 0.1 N NaOH was added to the pellet, and it was centrifuged for 30 minutes. To 0.5 ml of the extract, 5 ml of copper reagent ‘C’ was added (Reagent C: Mixture of reagents ‘A’ and ‘B’ in the ratio 50:1 ratio. Reagent A: 2 percent Na2CO3 in 0.1 N NaOH; Reagent B: equal volume of 1 percent CuSO4 and two percent sodium potassium tartrate). The absorbance was read at 550 nm in a spectrophotometer against an appropriate blank, Bovine Serum Albumin (BSA) was used as the standard.
Malondialdehyde (MDA) assay: Peroxidation of membrane lipids was measured based on the formation of malondialdehyde complex with Thiobarbituric acid, using Heath and Packer (1968). Take 200 mg leaf segment was homogenized in 3 ml of 50 mM phosphate buffer (pH 7.0) and then centrifuged at 8000 g in a Remi centrifuge (R-8C) for 20 minutes. Then, 2 mL of 0.5 percent TBA in 20 percent TCA solution was added to the 0.5 ml of remaining supernatant. The mixture was warmed in the water bath at 90°C for 30 minutes, followed by rapid cooling. Then the mixture was read spectrophotometrically at 532 nm and 600 nm wavelength using spectrophotometry.
Superoxide Dismutase (SOD) activity: The SOD activity was determined using Beauchamp and Fridovich’s (1971) method. The SOD activity was determined by the ability of this enzyme to retard the reduction of Nitro Blue Tetrazolium (NBT) by superoxide free radicals, which were generated in the reaction medium through photoreduction of riboflavin. The reaction mixture consists of 1 mL of buffer, I M sodium bicarbonate, 300 mM EDTA, 200 mM methionine, 100 μL of enzyme extract and 600 μM of riboflavin in test tube. The test tube was shaken and was placed 30 cm from light back consisting of six 15 W fluorescent lamps. The reaction was run for 10 min and was stopped by switching off the light. The optical density was determined at 560 nm, and the SOD activity results were calculated as μmol/mg Prot./min.
Catalase (CAT) activity: The CAT activity was determined based on the rate of H2O2 disappearance as measured by the decrease in absorbance at 240 nm, based on the method of Aebi (1974). The reaction mixture included phosphate buffer (50 mM, pH 7.0), H2O2 (3%), and 10 μL enzyme extract. The results were calculated as μ mol/mg Prot./min.
Statistical analysis: The results presented are the means ± standard error of five replicates (n=5). The results were statistically confirmed by Analysis Of Variance (ANOVA). Tukey’s HSD test was applied to find means significantly different from each other at p ≤ 0.05 level. The means that are not sharing a letter is significantly different at P ≤ 0.05 significance level.
Results and Discussion
Plant growth attributes
The results presented in Tab. 1 indicate that all the growth parameters of the selected plant were significantly (p<0.005) affected by NaCl treatment. However, SA application increased the growth characteristics compared to the control group. All the concentrations of SA increased the growth characteristics compared to T0 under non-saline conditions. However, among the various concentrations 0.5 mM concentration of SA was most effective in alleviating the harmful effects of salt stress. The combination of salt stress with SA treatment led to a significant (p<0.05) growth improvement compared to the salinity treatment alone. This increase remained important for the plants treated with 0.5 mM SA, which demonstrated increases of approximately 26.57%, 14.58%, 49.85%, 28.27%, 50.00%, 23.62% for the Root Length (RL), Shoot Length (SL), Fresh Weight Root (FWR), Fresh Weight Shoot (FWS), Dry Weight Root (DWR), and Dry Weight Shoot (DWS) compared to T1, respectively. Under saline conditions, by increasing concentration beyond 0.5 mM, the enhancement was not profound when compared to the T5 group.
Table 1. Effect of varying concentration of SA treatment and salt stress on the growth parameters of 567 black gram genotype. Values are means ± SE (n=5). Different letters indicate a significant difference at p<0.05.
| Treatment | Root length (cm/plant) | Shoot length (cm/plant) | Fresh weight root (g/plant) | Fresh weight shoot (g/plant) | Dry weight root (g/plant) | Dry weight shoot (g/plant) |
|---|---|---|---|---|---|---|
| Control | 12.05 0.00c | 24.12 0.014c | 5.24 0.014d | 8.15 0.007d | 2.91 0.028d | 3.95 0.028d |
| T1 (75 mM NaCl) | 9.22 0.035f | 20.85 0.007g | 3.41 0.035h | 6.26 0.035g | 1.86 0.014f | 3.09 0.014f |
| T2 (0.5 mM SA) | 15.32 0.021a | 28.18 0.015a | 7.83 0.021a | 11.49 0.021a | 4.44 0.035a | 5.65 0.035a |
| T3 (0.75 mM SA) | 13.41 0.028b | 26.04 0.022b | 6.43 0.007b | 10.67 0.028b | 3.87 0.042b | 4.89 0.014b |
| T4 (1.00 mM SA) | 12.11 0.014c | 23.32 0.035e | 5.93 0.042c | 9.74 0.014c | 3.87 0.042b | 4.12 0.028c |
| T5 (75+0.5 mM SA) | 11.67 0.042d | 23.89 0.028d | 5.11 0.014e | 8.03 0.042d | 2.79 0.021d | 3.82 0.042d |
| T6 (75+0.75 mM SA) | 11.21 0.05e | 22.43 0.043e | 4.28 0.014f | 7.53 0.049e | 2.14 0.007e | 3.51 0.05e |
| T7 (75+1.00 mM SA) | 9.32 0.014f | 21.16 0.021f | 3.96 0.049g | 6.87 0.014f | 1.97 0.014f | 3.21 0.014f |
Photosynthetic pigments
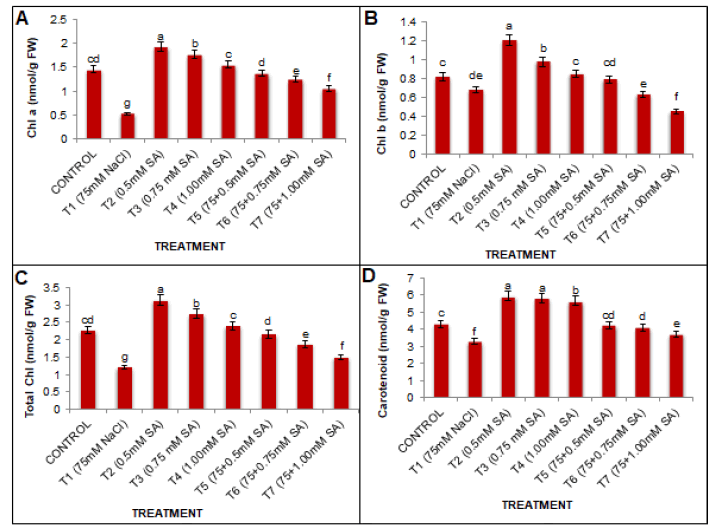
Salinity stress shows reciprocal relationship with photosynthetic pigments. Chlorophyll (a, b, total) and carotenoid contents were decreased by 63.44%, 17.07%, 87.67%, and 24.12% at 75 mM NaCl concentration over control group (Fig. 1). Under unstressed conditions salicylic acid significantly enhanced chlorophyll contents compared to water-sprayed plants. The highest significant value was obtained with SA at 0.5 mM under non-saline control conditions. There was no positive response by increasing the concentration of SA beyond 0.5 mM. The combination of salt stress with SA treatment at 0.5 mM was effective in mitigating the negative effects of salt stress led to a significant (p<0.05) improvement in photosynthetic parameters compared to the salinity treatment alone.
Figure 1: A): Stress-induced ethylene production by soybean plants without seed bacterization; B): inoculation with rhizobia of strain 604k; C) Tn5 mutant B1-20 in the stages of cotyledons (I), primordial leaves (II) and the first true leaf (III) ); (x ± SD, n=10).
Total soluble protein content
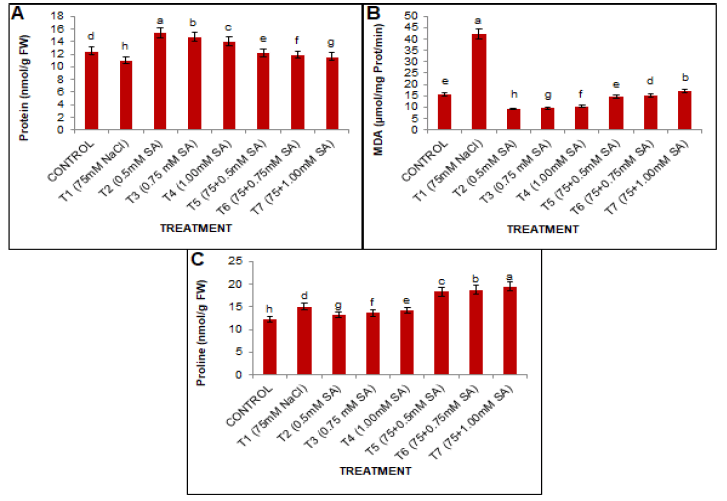
As presented in Fig 2A, the comparison of means indicated that all treatments were significantly different. The results demonstrated that salt stress significantly decreased the protein content compared to the conditions without salinity. This protein content was reduced by 11.54% at T1 (75 mM NaCl) when compared with T0, respectively. However, SA application ameliorates the harmful impacts of salt treatment. The combination of salt stress and SA treatment (0.5 mM) was found to be effective in alleviating the harmful effects of salt stress and led to an increase of 10.87% compared to the T1 treatment group.
Figure 2: Effects of SA and salt stress on (A) total protein contents, (B) MDA contents, and (C) proline contents in black gram leaves. The obtained values were analyzed by using one-way analysis of variance (ANOVA)-using SPSS 16.0 software. Different letters indicate a significant difference between the mean (±SE) of treatments (n=5) at P<0.05 significant level.
Lipid peroxidation
Plants subjected to salt stress showed an increased content of MDA in black gram leaves Fig. 2B Damage to cell membranes was studied by estimating the MDA content in leaves. The current results presented a significant increase of MDA content in the stressed plants compared to the unstressed plants. However foliar application of SA reduced the MDA content in the study plant over the control group. MDA accumulation was reduced by SA treatment in unstressed plants. However, 0.5 mM SA application was found to be most effective in T5, which reduced the MDA content by 65.61% compared to T1.
Proline content
The present results showed that salt stress treatment caused significant leaf proline accumulation in the leaves of stressed plants over non-saline control during plant growth. Exogenous application of SA caused maximum accumulation of proline in salt-stressed plants (Fig. 2C). However, with the increase in the concentration of SA, proline content also increased, and a 28.86% maximum increase was observed in plants treated with 1.00 mM SA under salt stress compared to T1 treatment groups.
Catalase activity
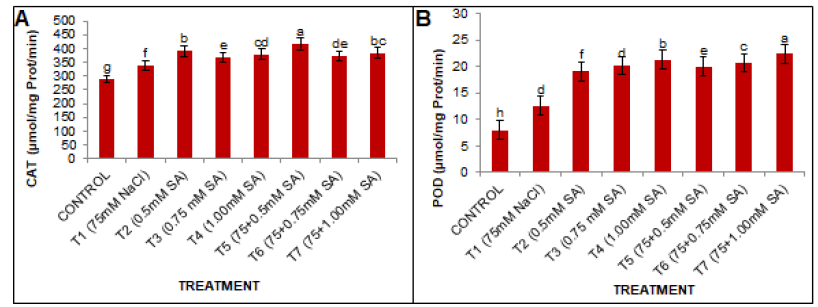
CAT activity was significantly (p<0.005) affected by NaCl application (Fig. 3A). At 75 mM NaCl 16.60% increase in CAT activity was observed in the T1 treatment over the control group. However, the exogenous application of various SA concentrations enhanced the CAT activity in the study plant under non-saline conditions. Among various concentrations of SA, 0.5 mM was found to be the most effective and to 24.03% high CAT activity compared to the T1 treatment group.
Figure 3: Effects of SA and salt stress on (A) CAT and (B) POD activities in black gram leaves. The obtained values were analyzed by using one-way Analysis of Variance (ANOVA)-using SPSS 16.0 software. Different letters indicate a significant difference between the mean (± SE) of treatments (n=5) at P<0.05 significant level.
Peroxidase activity
POD activity was significantly affected by NaCl application. The data presented in (Fig. 3b) indicate that the POD activity was increased by 56.66% at 75 mM NaCl significantly (p<0.05) as compared to the control group. Under various SA treatments for the unstressed plants (approximately 138.24%, 151.30%, and 165.62% for the T2, T3, and T4 treatments, respectively) compared to the control group. Furthermore, in response to the salt stress, the POD activity significantly increased compared to conditions without stress. However, the application of SA, i.e. 1.00 mM, was found to be most under salt stress and enhanced the POD activity by 77.90% for T7, compared to T1.
Discussion
Salinity is one of the challenging environmental factor affecting plant growth and development aspects. Excessive uptake and accumulation of ions, particularly sodium (Na+) and chloride (Cl-), causes osmotic stress and cytotoxicity, restricting plant growth and productivity. Several Physiological and molecular processes govern osmotic and ionic stress tolerance by enhancing antioxidant activity, osmolyte buildup, and ion equilibrium (Roussos et al., 2013; Isayenkov and Maathuis 2019; Saddiq et al., 2021). Our present study shows that salt stress retards the plant growth in terms of root length, shoot length, fresh weight, dry weight, and biomass exclusively by excessive uptake of Na+ and Cl- ions and antioxidant activity of Vigna mungo L. plants under salt stress. The results indicate that the exposure of black gram plants to salt stress negatively affected their growth. The plants, under saline water irrigation (75 mM NaCl), demonstrated a decrease in all studied growth parameters. These results are similar to those documented in several plant species, such as wheat (Abdel-Lattif et al., 2019), soybeans (Ghassemi-Golezani et al., 2018), and mung beans (Salingpa et al. 2018). According to Acosta-Motas et al., (2017), decline in growth is a key aspect for depicting the extent of salt stress-led devastation independent of type of species. Ahmad et al. (2016) suggested that salt stress-induced suppression of cell division and cell elongation is the key cause of reduced growth and biomass and our results also showed higher sensitivity of Vigna mungo to salinity. SA-induced advantageous effect on growth characteristics could be endorsed to reduced Na+ and Cl– uptake, ethylene synthesis inhibition, or increased uptake of essential nutrients, such as K+, Ca2+, Mg2+, Fe, Mn, N, and Cu2+ (Ghassemi-Golezani et al., 2018). These measures can help plants thrive well by reducing ionic and osmotic stress. Exogenous application of SA can modulate stomatal opening, promote CO2 assimilation, and reduce transpirational water loss under saline circumstances (Ribeiro et al., 2020). As a result, the plants could maintain their turgor, boost their photosynthetic capacity and increase their yield (Fghire et al., 2015; Ribeiro et al. 2020).
Photosynthesis, the prime photosynthetic machinery, gets easily targeted by salt stress. Chlorophyll (a, b, total) and carotenoid contents decreased with increasing NaCl concentration (Fig. 1). Under unstressed conditions, salicylic acid significantly enhanced chlorophyll contents compared to water-sprayed plants. The highest significant values were obtained with salicylic acid at 0.5 mM under non-saline conditions. The combination of salt stress with SA treatment led to a significant (p<0.05) improvement in photosynthetic parameters compared to the salinity treatment alone. The present study results agree with the findings of earlier researchers where salt-induced reduction in the chlorophyll contents is alleviated by the foliar application of salicylic acid in crops such as tomatoes (Shahba et al., 2010). Exogenous SA application protects chlorophyll from degradation by regulating antioxidant molecules synthesis, suppressing genes involved in the senescence process, up-regulating chlorophyll biosynthesis genes, and down-regulating chlorophyll degradation genes (Altaf et al., 2021). SA plays a pivotal role in chloroplast biosynthesis, maintaining chlorophyll stability, regulates photosynthesis by guarding this vital organelle against toxic effects of ROS to survive under salt stress. The oxidation of chlorophyll and other chloroplast pigments, as well as the instability of the pigment-protein complex under salt stress, could explain the decrease in chlorophyll concentration in saltaffected black gram genotypes Stępień and Kłbus (2006).
Surprisingly, foliar applications of SA significantly neutralized the salt injuries and maintained a high concentration of carotenoids in salt-stressed plants (Fig. 4). The decomposition of beta carotene and the zeaxanthin production in the xanthophyll cycle results decrease in carotenoid content under stress conditions (Sultana et al., 1999). A similar observation was also reported by Arnao and Hernandez-Ruiz (2019); Bukhat et al., (2020); Faraz et al., (2020) that SA could regulate steps in carotenoid biosynthesis and influence cellular signaling, and trigger redox-sensitive regulatory pathways. Salinity stress increases the concentrations of growth regulators such as abscisic acid and ethylene that activate Chlorophylase, causing break down of chlorophyll.
The stressed plants showed decreased protein content compared to the control group. However, there was an increase in the protein content in SA-treated plants under saline conditions. Similar results have been found with wheat (Alsahli et al., 2019), tomatoes (Arbaoui and Belkhodja, 2018), and faba beans (Anaya et al., 2017). Salt stress negatively affects protein biosynthesis by assisting protein degradation. A decrease in soluble protein level of salt-treated Vigna mungo may be possibly due to catabolism of proteins under salt stress. The salt stress condition could affect different stages of nitrogen metabolism, such as absorption, ionic reduction, and protein synthesis. Under stress conditions, plant produce several proteins that can regulate intracellular signaling and gene expression related to critical metabolic processes (Alsahli et al., 2019). Arbaoui and Belkhodja (2018) and Rajeshwari and Bhuvaneshwari (2017) have explained that the application of SA under abiotic and biotic stresses can improve plant tolerance by protecting proteins ultrastructure and increasing metabolic enzyme activity.
The oxidative reactions induce a sequence of reactions that lead to membrane peroxidation. In this work, the salt stress resulted in a significant increase in MDA content in black gram leaves due to oxidative stress (Tab. 1). This is supported by a positive correlation between H2O2 and MDA (Moustafa-Farag et al., 2017). Similar reports have been provided for Arabidopsis thaliana L. by Yu et al., (2020). However, under saline stress conditions, the treatment with SA remarkably reduced the MDA accumulation in the treated plants compared to those under salinity treatment alone. Similar results have been found by Torun (2019) in barley plants and Wang et al., (2018) in wheat plants; those studies indicated that exogenous SA treatment could reduce the higher MDA levels caused by salt and cold stress, respectively. The findings suggest that SA effectively limits cell membrane damage through the antioxidant defence system. Indeed, SA application ameliorated the plants’ stress tolerance by reducing membrane oxidation (Wang et al., 2018), protecting cellular components, and strengthening cell walls (Ahanger et al., 2020).
SA treatment and salt stress in T5 increased proline accumulation by 17.69% compared to T1. A similar increase in proline by SA treatment has earlier been observed in Panax (Ali et al., 2007), Tomato (Mimouni et al., 2016), and Cucumber (Youssef et al., 2018). To protect thylakoidal protein complexes, salt stress stimulates the production of reactive oxygen species in the chloroplast as well as the accumulation of several osmoprotectants and antioxidants. Proline is one of the potential osmoprotectant with roles in osmotic regulation, protection of PSII activity, antioxidant activity, and cell membrane stabilization (Szabados and Savouré, 2010).
In the present study, salt stress enhances the antioxidant activity in the studied plant. The increase in antioxidant enzyme activity was highly significant in SA-treated plants under saline conditions. The foliar spray with SA significantly increased POD and CAT activity which is inversely related to MDA and H2O2, respectively. SA foliar spray ameliorated the salt-induced oxidative damage to PSII by modulating the activities of antioxidant enzymes. These findings are explained by Bose et al. (2017), who claim that SA triggers numerous pathways in the chloroplast to scavenge ROS and protect the photosynthetic apparatus, including a battery of antioxidant enzymes. The enhancement of salt tolerance against oxidative stress is usually related to the improving antioxidant enzyme activities in plants (Torun 2019). This increase in antioxidant enzyme activity was highly significant in SA-treated plants under saline conditions. Several recent studies have proven SA’s significance in activating antioxidant enzymes under various stress conditions both directly and indirectly. Cai et al., (2015) investigated the effects of SA treatment on the antioxidative system. They found that the jasmine plant shows greater resistance to cold stress, which was likely linked to higher antioxidant enzyme activity, which resulted in decreased ROS generation, and altered gene expression. Other similar studies have found that SA improved antioxidant enzyme activity such as POD and SOD, when applied exogenously in black gram (Solanki Mital et al. 2018). According to Faried et al. (2017), SA ability to bind with antioxidant enzymes (POD and CAT) involved in ROS metabolism and redox homeostasis explains its beneficial effect on the antioxidant system of salt-stressed potato plants.
Conclusion
To summarize, our results clearly show that saline circumstances have a detrimental effect on the morphological, biochemical, and antioxidant characteristics of black gram by retarding growth, disrupting photosynthetic machinery, accumulating ROS, and modifying nutrient absorption and translocation. On the other hand, SA application alleviates the above-mentioned detrimental effects of salt stress by increasing osmolyte buildup, antioxidant machinery, and its effect on Na+ and K+ uptake, which is a prerequisite for metabolic processes. SA application not only nullified the impacts of salt stress but also enhanced the photosynthetic function and growth. However, the mode of action of SA in remedying the harmful impacts depends on the effective concentration. Notably, 0.5 mM SA was found to be most effective in mitigating the harmful impacts of stress, as higher concentrations were inhibitory in most cases. As a result, it is fair to conclude that foliar SA exerted stimulatory effects on the black gram genotype when exposed to salt. Our study opens a new research window that might bring revolution in the global agriculture sector and provide new insights into the application of salicylic acid to enhance tolerance to salt stress and minimize crop production yield losses under field environments.
Declaration of Competing Interest
The authors declare that they have competing interests.
References
Abdel-Lattif H.M., Abbas M. S., Taha M.H. (2019). Effect of salicylic acid on productivity and chemical constituents of some wheat (Triticum aestivum L.) varieties grown under saline conditions. JAPS 29: 1054-1064.
Aebi H. (1984). Catalase in vitro. Methods in Enzymology 105: 121-126.
Ahanger M.A., Aziz U., Alsahli A.A., Alyemeni M.N., Ahmad P. (2019). Influence of exogenous salicylic acid and nitric oxide on growth, photosynthesis, and ascorbate-glutathione cycle in salt stressed Vigna angularis. Biomolecules 10: 42.
Ahmad P., Ahanger M.A., Alyemeni M.N., Wijaya L., Egamberdieva D., Bhardwaj R., Ashraf M. (2017). Zinc application mitigates the adverse effects of NaCl stress on mustard [Brassica juncea (L.) Czern & Coss] through modulating compatible organic solutes, antioxidant enzymes, and flavonoid content. J Plant Interactions 12: 429-437.
Ali M.B., Hahn E.J., Paek K.Y. (2007). Methyl jasmonate and salicylic acid induced oxidative stress and accumulation of phenolics in Panax ginseng bioreactor root suspension cultures. Molecules 12: 607-621.
Alsahli A., Mohamed A.K., Alaraidh I., Al-Ghamdi A., Al-Watban A., El-Zaidy M., Alzahrani S.M. (2019). Salicylic acid alleviates salinity stress through the modulation of biochemical attributes and some key antioxidants in wheat seedlings. Pak J Bot 51: 1551-1559.
Altaf M.A., Shahid R., Ren M.X., Altaf M.M., Khan L.U., Shahid S., Jahan M.S. (2021). Melatonin alleviates salt damage in tomato seedling: A root architecture system, photosynthetic capacity, ion homeostasis, and antioxidant enzymes analysis. Scientia Horticulturae 285: 110145.
Anaya F., Fghire R., Wahbi S., Loutfi K. (2017). Antioxidant enzymes and physiological traits of Vicia faba L. as affected by salicylic acid under salt stress. J Mater Environ Sci 8: 2549-2563.
Arbaoui M., Belkhodja M. (2018). Metabolic response of tomato (Lycopersicon esculentum Mill.) under salt stress combined with hormones. Int J Agron Agri Res 12: 37-45.
Arnao M.B., Hernández-Ruiz J. (2019). Melatonin: a new plant hormone and/or a plant master regulator?. Trends in Plant Science 24: 38-48.
Arnon D.I. (1949). Copper enzymes in isolated chloroplasts. Polyphenoloxidase in Beta vulgaris. Plant Physiology 24: 1.
Arulbalachandran D., Ganesh K.S., Subramani A. (2009). Changes in metabolites and antioxidant enzyme activity of three Vigna species induced by NaCl stress. American-Eurasian J Agron 2: 109-116.
Athar H.R., Ashraf M. (2009). Strategies for crop improvement against salinity and drought stress: An overview. Salinity Water Stress 1-16.
Bastam N., Baninasab B., Ghobadi C. (2013). Improving salt tolerance by exogenous application of salicylic acid in seedlings of pistachio. Plant Growth Regulation 69: 275-284.
Bates L.S., Waldren R.P., Teare I.D. (1973). Rapid determination of free proline for water-stress studies. Plant Soil 39: 205-207.
Beauchamp C.O., Fndovich I. (1971). Superoxide dismutase: improved assays and an assayapplicableto acrylamidegels. Anal Biochem 44: 276-287.
Google Scholar Cross Ref
Bose J., Munns R., Shabala S., Gilliham M., Pogson B., Tyerman S.D. (2017). Chloroplast function and ion regulation in plants growing on saline soils: lessons from halophytes. J Experimental Bot 68: 3129-3143.
Bukhat S., Manzoor H., Athar H.U.R., Zafar Z.U., Azeem F., Rasul S. (2020). Salicylic acid induced photosynthetic adaptability of Raphanus sativus to salt stress is associated with antioxidant capacity. J Plant Growth Regulation 39: 809-822.
Cai H., He M., Ma K., Huang Y., Wang Y. (2015). Salicylic acid alleviates cold-induced photosynthesis inhibition and oxidative stress in Jasminum sambac. Turk J Biol 39: 241-247.
da Silva Ribeiro J.E., Vieira de Sousa L., Iarley da Silva T., Silva Nóbrega J., Andrade Figueiredo F.R., Alcântara Bruno R.D.L., Bandeira de Albuquerque M. (2020). Citrullus lanatus morphophysiological responses to the combination of salicylic acid and salinity stress. Brazilian J Agricultural Sci 15.
Deinlein U., Stephan A.B., Horie T., Luo W., Xu G., Schroeder J.I. (2014). Plant salt-tolerance mechanisms. Trends Plant Sci 19: 371-379.
Eraslan F., Inal A., Gunes A., Alpaslan M. (2007). Impact of exogenous salicylic acid on the growth, antioxidant activity and physiology of carrot plants subjected to combined salinity and boron toxicity. Scientia horticulturae 113: 120-128.
Es-sbihi F.Z., Hazzoumi Z., Aasfar A., Amrani Joutei K. (2021). Improving salinity tolerance in Salvia officinalis L. by foliar application of salicylic acid. Chem Biolog Technol Agricul 8: 1-12.
Faraz A., Faizan M., Sami F., Siddiqui H., Hayat S. (2020). Supplementation of salicylic acid and citric acid for alleviation of cadmium toxicity to Brassica juncea. J Plant Growth Regulation 39: 641-655.
Fariduddin Q., Khan T.A., Yusuf M., Aafaqee S.T., Khalil R.R.A.E. (2018). Ameliorative role of salicylic acid and spermidine in the presence of excess salt in Lycopersicon esculentum. Photosynthetica 56: 750-762.
Berlinet C., Brat P., Brillouet J.M., Ducruet V. (2006). Ascorbic acid, aroma compounds and browning of orange juices related to PET packaging materials and pH. J Sci Food Agricul 86: 2206-2212.
Fghire R., Anaya F., Ali O.I., Benlhabib O., Ragab R., Wahbi S. (2015). Physiological and photosynthetic response of quinoa to drought stress. Chil J Agric Res 75: 174-183.
Ghassemi G.K., Farhangi A.S., Bandehagh A. (2018). Salicylic acid and jasmonic acid alter physiological performance, assimilate mobilization and seed filling of soybean under salt stress. Acta Agric Slov 111: 597-607.
Hasanuzzaman M., Bhuyan M.H.M., Zulfiqar F., Raza A., Mohsin S.M., Mahmud J.A., Fotopoulos V. (2020). Reactive oxygen species and antioxidant defense in plants under abiotic stress: Revisiting the crucial role of a universal defense regulator. Antioxidants 9: 681.
Hayat Q., Hayat S., Irfan M., Ahmad A. (2010). Effect of exogenous salicylic acid under changing environment: a review. Environ Exp Bot 68: 14-25.
Chen H., Bullock Jr D.A., Alonso J.M., Stepanova A.N. (2021). To fight or to grow: the balancing role of ethylene in plant abiotic stress responses. Plants 11: 33.
Dubois M., Van den Broeck L., Inzé D. (2018). The pivotal role of ethylene in plant growth. Trends in Plant Sci 23: 311-323.
Husain T., Fatima A., Suhel M., Singh S., Sharma A., Prasad S.M., Singh V.P. (2020). A brief appraisal of ethylene signaling under abiotic stress in plants. Plant Signal Behav 15: 1782051.
Heath R.L., Packer, L. (1968). Photo peroxidation in isolated chloroplasts: I. Kinetics and stoichiometry of fatty acid peroxidation. Arch Biochem Biophys 125: 189-198.
Hoang H.L., de Guzman C.C., Cadiz N.M., Hoang T.T.H., Tran D.H., Rehman H. (2020). Salicylic acid and calcium signaling induce physiological and phytochemical changes to improve salinity tolerance in red amaranth (Amaranthus tricolor L.). J Soil Sci Plant Nutr 20: 1759-1769.
Khan M.I.R., Fatma M., Per T.S., Anjum N.A., Khan N.A. (2015). Salicylic acid induced abiotic stress tolerance and underlying mechanisms in plants. Front Plant Sci 6: 462.
Iqbal M., Saliha I., Nadeem M., Tatheer F., Itrat A.B. (2018). Salinity effects on wheat (Triticum aestivum L.) characteristics. Int J Biosci 12: 131-146.
Isayenkov S.V., Maathuis F.J. (2019). Plant salinity stress: many unanswered questions remain. Front Plant Sci 10: 80.
Khan M.I.R., Iqbal N., Masood A., Khan N.A. (2012). Variation in salt tolerance of wheat cultivars: role of glycinebetaine and ethylene. Pedosphere 22: 746-754.
Lowry O.H., Rosebrough N.J., Farr A.L., Randall R.J. (1951). Protein measurement with the Folin phenol reagent. J Biol Chem 193: 265-275.
Meloni D.A., Gulotta M.R., Martínez C.A., Oliva M.A. (2004). The effects of salt stress on growth, nitrate reduction and proline and glycinebetaine accumulation in Prosopis alba. Braz J Plant Physiol 16: 39-46.
Mimouni H., Wasti S., Manaa A., Gharbi E., Chalh A., Vandoorne B., Lutts S., Ahmed H.B. (2016). Does salicylic acid (SA) improve tolerance to salt stress in plants? A study of SA effects on tomato plant growth, water dynamics, photosynthesis, and biochemical parameters. OMICS 20: 180-190.
Nazar R., Umar S., Khan N.A. (2015). Exogenous salicylic acid improves photosynthesis and growth through increase in ascorbate-glutathione metabolism and S assimilation in mustard under salt stress. Plant Signal Behav 10: e1003751.
Neocleous D., Vasilakakis M. (2007). Effects of NaCl stress on red raspberry (Rubus idaeus L.‘Autumn Bliss’). Sci Hortic 112: 282-289.
Rajeshwari V., Bhuvaneshwari V. (2017). Salicylic acid induced salt stress tolerance in plants. Internat J Plant Biol Res 5: 1067-1073.
Roussos P.A., Gasparatos D., Kyriakou C., Tsichli K., Tsantili E., Haidouti C. (2013). Growth, nutrient status, and biochemical changes of sour orange plants subjected to sodium chloride stress. Commun Soil Sci Plant Anal 44: 805-816.
Raza A. (2022). Plant biotechnological tools: Solutions for raising climate resilient crop plants. Modern Phytomorphol 15: 132-133.
Google Scholar Cross Ref
Salingpa T.W., Lal E.P., Shukla P.K. (2018). Effect of foliar application of salicylic acid on growth, yield, physiological and biochemical characteristics of mung bean (Vigna radiata L.) under salt stress. J Pharmacogn Phytochem 7: 1857-1860.
Saddiq M.S., Afzal I., Iqbal S., Hafeez M.B., Raza A. (2021). Low leaf sodium content improves the grain yield and physiological performance of wheat genotypes in saline-sodic soil. Pesqui Agropecu Bras 51.
Sabagh A.E., Mbarki S., Hossain A., Iqbal M.A., Islam M.S., Raza A., Llanes A., Reginato M., Rahman M.A., Mahboob W., Singhal R.K., Kumari A., Rajendran K., Wasaya A., Javed T., Shabbir R., Rahim J., Barutçular C., Ur Rahman M.H., Raza M.A., Ratnasekera D., Konuskan Ö.l., Hossain M.A., Meena V.S., Ahmed S., Ahmad Z., Mubeen M., Singh K., Skalicky M., Brestic M., Sytar O., Karademir E., Karademir C., Erman M. Farooq M. (2021). Potential Role of Plant Growth Regulators in Administering Crucial Processes Against Abiotic Stresses. Front Agron 3: 648694
Schubert S., Neubert A., Schierholt A., Sümer A., Zörb, C. (2009). Development of salt-resistant maize hybrids: the combination of physiological strategies using conventional breeding methods. Plant Sci 177: 196-202.
Shahba Z., Baghizadeh A., Yosefi M. (2010). The salicylic acid effect on the tomato (Lycopersicum esculentum Mill.) germination, growth and photosynthetic pigment under salinity stress (NaCl). J Stress Physiol Biochem 6.
Shamili M., Ghalati R.E., Samari F. (2021). The impact of foliar salicylic acid in salt-exposed guava (Psidium Guajava L.) seedlings. Int J Fruit Sci 21: 323-333.
Sharma M., Kumar P., Verma V., Sharma R., Bhargava B., Irfan M. (2022). Understanding plant stress memory response for abiotic stress resilience: Molecular insights and prospects. Plant Physiol Biochem 179: 10-24.
Shaukat K., Zahra N., Hafeez M.B., Naseer R., Batool A., Batool H., Raza A., Waheed A. (2022). Role of salicylic acid induced abiotic stress tolerance and underlying tolerance in plants. In Emerging Plant Growth Regulators in Agriculture pp: 73-98.
Solanki Mital V., Trivedi Sandhya K., Kandoliya U. K., Golakiya B. A. (2018). Effect of exogenous application of salicylic acid on antioxidative enzymes in black gram (Vigna mungo (L.) Hepper) irrigated with saline water. Int J Chem Stud 6: 2107-2116.
Stepien P., KÅ?bus G. (2006). Water relations and photosynthesis in Cucumis sativus L. leaves under salt stress. Biol Plant 50: 610-616.
Sultana N., Ikeda T., Itoh R. (1999). Effect of NaCl salinity on photosynthesis and dry matter accumulation in developing rice grains. Environ Exp Bot 42: 211-220.
Szabados L., Savoure A. (2010). Proline: a multifunctional amino acid. Trends Plant Sci 15: 89-97.
Talaat N.B. (2019). Role of reactive oxygen species signaling in plant growth and development. React Oxyg Nitrogen Sulfur Species Plants Prod Metab Signal Def Mech 225-266.
Torun H. (2019). Timeâ?course analysis of salicylic acid effects on ROS regulation and antioxidant defense in roots of hulled and hulless barley under combined stress of drought, heat and salinity. Physiol Plant 165: 169-182.
Wang W., Wang X., Huang M., Cai J., Zhou Q., Dai T., Jiang D. (2018). Hydrogen peroxide and abscisic acid mediate salicylic acid-induced freezing tolerance in wheat. Front Plant Sci 9: 1137.
Wani A.B., Chadar H., Wani A.H., Singh S., Upadhyay N. (2017). Salicylic acid to decrease plant stress. Environ Chem Lett 15: 101-123.
Khan A., Li R.J., Sun J.T., Ma F., Zhang H.X., Jin J.H., Gong Z.H. (2018). Genome-wide analysis of dirigent gene family in pepper (Capsicum annuum L.) and characterization of CaDIR7 in biotic and abiotic stresses. Sci Rep 8: 1-21.
Youssef S.M., Abd Elhady S.A., Aref R.M., Riad G.S. (2018). Salicylic acid attenuates the adverse effects of salinity on growth and yield and enhances peroxidase isozymes expression more competently than proline and glycine betaine in cucumber plants. Gesunde Pflanz 70: 75-90.
Yu L.L., Liu Y., Zhu F., Geng X.X., Yang Y., He, Z.Q., Xu F. (2020). The enhancement of salt stress tolerance by salicylic acid pretreatment in Arabidopsis thaliana. Biol Plant 64: 150-158.
Zafar Z.U., Manzoor H., Rasul S., Noreen S., Ali Q., Iqbal M., Ashraf M. (2017). Strategies to improve crop salt and drought tolerance: Success and limitations. Agrobios India 11: 265-298.
Zhao J., Ren W., Zhi D., Wang L., Xia G. (2007). Arabidopsis DREB1A/CBF3 bestowed transgenic tall fescue increased tolerance to drought stress. Plant Cell Rep 26: 1521-1528
Maas E.V., Hoffman G.J. (1977). Crop salt tolerance-current assessment. J Irrig Drain Div 103: 115-134.
Manjri Singh A., Gupta S.D., Bahadur R., Singh A.K. (2018). Responses of Blackgram (Vigna mungo) to Foliar Applied Plant Growth Regulators. Int J Curr Microbiol Appl Sci 7: 4058-4064.
Thakur V., Teggelli R.G., Meena M.K. (2017). Influence of foliar nutrition on growth and yield of pulses grown under north eastern dry zone of Karnataka: A Review. Int J Pure App Biosci 5: 787-795.