Research - Modern Phytomorphology ( 2025) Volume 19, Issue 4
Histochemistry and powder microscopy of Ceropegia juncea Roxb.
Bhuvaneswari Manoharan1*, Chitra Vadivu Chinnasamy1, Raja Kannan2 and Balakrishnan Veluchamy32Center for global health research, Saveetha Medical College and hospital, Saveetha Institute of Medical and Technical Sciences, Saveetha Nagar, Thandalam, Chennai, Tamil Nadu, India
3Department of Botany, Arignar Anna Government Arts College, Namakkal, Tamil Nadu, India
Bhuvaneswari Manoharan, PG and Research Department of Botany, Vellalar College for Women, Erode, India, Email: bhuvi.botany@gmail.com
Received: 06-Jun-2025, Manuscript No. mp-25-166267; Accepted: 06-Jul-2025, Pre QC No. mp-25-166267 (PQ); Editor assigned: 10-Jun-2025, Pre QC No. mp-25-166267 (PQ); Reviewed: 20-Jun-2025, QC No. mp-25-166267 (Q); Revised: 30-Jun-2025, Manuscript No. mp-25-166267 (R); Published: 12-Jul-2025, DOI: 10.5281/zenodo.17414588
Abstract
Introduction: The histology domain concerned with detecting the chemical components present in the cells and tissues is called histochemistry. The Apocynaceae member of Ceropegia juncea Roxb. containing rich sources of phytoconstituents.
Material and Methods: Histochemistry and powder microscopy of tuber examined with different chemicals for specified location of secondary metabolites.
Result: The C. juncea tuber containing Calcium oxalate crystals were found in the ground parenchyma. Starch grains are found to be large masses in the cortical parenchyma and most of the secondary metabolites found in the different cells of the tuberous root. Alkaloids can cure cancer, inflammation and protein used for weight loss, rich in antioxidants, anti-diabetic, improve immune system followed by lipids used to regulate hormone, transmit nerve impulses and store energy. Tannin has the ability of antioxidants, to maintain skin and anti-inflammatory.
Conclusion: Natural chemicals are localized from C. juncea tuber different cells to isolate phytoconstituents for further in- vitro and in- vivo studies to produce novel drug. The above-all characteristic features of C. juncea tuber was used to find out the purity of the herbal drug.
Keywords
Histochemical analysis, Phytoconstituents, Tissues, Apocynaceae
Introduction
Histochemistry is crucial for resolving fundamental biosystematics issues. Plants continue to devour an important influence on human health and rich in bioactive chemicals. India is known as the botanical garden of the globe because it contains the world’s largest source of medicinal plants. Medicinal plants have played an integral part in the sociocultural advancement of India’s rural populations. One of the fundamental sources of biologically active molecules is thought to be plants and their leaves, in many deprived nations, plant-based substances are still used extensively in basic medical care as therapeutic remedies. Herbal medications are safer than synthetic ones. Since, the phytochemicals in the plant extract target the biochemical mechanism. Medicinal herbs have been used for centuries to treat and prevent a variety of ailments, particularly in developing countries where access to contemporary medical facilities and services is restricted and infectious diseases are prevalent. Plant-based natural compounds present an opportunity to develop new drugs that are more secure, more potent and have superior pharmacological effects compared to synthetic (Arora and Meena, 2018). The histochemistry is an influential performance of localization of elements in biological tissues (Pearse, 1988, Krishnamurthy, 1998). Histochemical practices have been active to illustrate configuration and progress and to study time development of confession and dissemination of foremost stowage compounds such as protein, lipid, starch, crystals and tannin (Krishnan, et al. 2001, Krishnan and Dayanandan, 2003).
Modern Pharmacopoeias of herbal drugs are included with microscopical descriptions in new Monographs (Alamgir, 2017). Histology is used to identify the localization of the chemical compounds in specific cells or tissues (Kadam, et al. 2013). The most used technique for validating herbal medications is powder microscopy. Cellular characteristics can be used to determine the botanical source and quality (Singh, et al. 2018).
Ceropegia genus with 200 species present throughout the World also has distribution in tropical and subtropical Asian countries (Pradeepika, et al. 2018). In India the use of plants for the medicinal treatment dates to Vedic era (Karayil, et al. 2014). In India more than 55 species have been reported, few species are edible and few species are domesticated (Pradeepika, et al. 2018). The Ceropegia juncea Roxb. proven the phytoconstituents are ability to cure anti-diabetic, anti-ulcer, hypertensive and anti-ulcer.
Materials and Methods
Chemicals
The diagnostic mark chemicals were procuring for this study from the HI–MEDIA Pvt. Ltd., Mumbai.
Collection of study plant
The fresh and healthy plant of Ceropegia juncea Roxb. was collected from the wasteland of Pudhukanelli (Latitude-10.93939594°N and Longitude 78.2818°E), Pavithram, Karur District and also the particular specimen was identified in the Bodha Hills, Rasipuram, Namakkal District (Latitude-11.5476°N and Longitude 77.95904248°E). The collected specimen was identified using the “Flora of the Presidency of Madras” and authenticated by the “Botanical Survey of India”, Southern Circle with the reference No. BSI/SRC/5/23/2021/Tech/184 and the voucher specimen were deposited in the PG and Research Department of Botany, Vellalar College for Women (Autonomous), Thindal, Erode.
Microscopic analysis
Histochemical examination: The free hand sections of tuberous root of C. juncea treated with Dragendorff’s reagent, Wagner’s reagent, Sudan red III and IV, Ruthenium red, neutral red, Ferric chloride, coo massive Brilliant Blue (CBB), Phloroglucinol+50% HCl, Iodine water solution, Iodine Potassium Iodide (IKI) and for 2-5 minutes alone left the dose for the documentation of the explicit cellular area. Eliminate the excessive stains by corresponding solvents equestrian with glycerin and covered with cover glass and margins were wrapped with nail polish and perceived under microscope (Krishnamurthy, 1998).
Powder microscopy: The C. juncea tuberous root was rinsed with water to confiscate muck. The C. juncea tuberous root was cut into pieces and dried at room temperature for 10-15 days. Then the sample was transferred to a mixer a grinder for pulverizing into a coarse powder. For further analysis, the sample was stored in air-tight fine quality plastic containers at room temperature. For further analysis, the sample was stored in air-tight fine quality plastic containers at room temperature (Harborne, 1973).
Powder characteristics: The C. juncea little amount of tuber powder was used to remove with chloral hydrate solution and blemished with hydrochloric acid, phloroglucinol and a limited cases of Iodine were also used for discoloration. The tainted powder was equestrian in glycerine for miniscule observation (Kokate, 1990). Typescripts were observed under Axiolab 5 trinocular microscope fitted with Axiocam 208 color digital camera under bright field. Photomicrographs of diagnostic characters were apprehended and predictable
Results
Histochemical localization of secondary metabolites in the tuberous root of Ceropegia juncea Roxb.
Alkaloids appear reddish-orange when stained with Dragendorff’s reagent and the cells appear to be a thick solid piece (Fig. 1.a). Isolated bodies of Calcium oxalate crystals were found in the ground parenchyma (Fig. 1.e). Lipid is stained with a neutral red and appears dark red in colour. Lipid appears in thick, solid fragments (Fig. 1.c). Tannin appears brownish black and stained with Fecl2 and abundant in the cortical parenchyma (Fig. 1.d). Protein appears dark blue and stained with CBB and found to be in the cortical cells (Fig. 1.b). The starch grains are stained with Iodine Potassium Iodide (IKI), which is black in colour. Starch grains are found to be large masses in the cortical parenchyma (Fig. 1) (Tab. 1). Steroid is visible by adding chloroform, sulphuric acid and acetic acid, green colour which is present in the cortex (Fig. 1.h).Phenol is addition with Anhydrous Ferric chloride +90% Ethanol showing of Brown to black indicates the present in the Epidermis and Vascular bundle (Fig. 1.i).Flavonoid by accumulation 25% lead acetate exhibits the yellow colour specifies the present in the Epidermis and Vascular bundle (Fig.1j) (Tab. 1).
Figure 1. Histochemical localization of secondary metabolites in the tuber of Ceropegia juncea Roxb. Note: Co: Cortex; Ep: Epidermis; Vb: Vascular bundle.
| S. No | Secondary metabolites | Reagents | Results obtained | Tuberous root |
|---|---|---|---|---|
| 1 | Alkaloid | Dragendorff’s Reagent | Reddish orange | Cortical tissue |
| 2 | Protein | Coo massive Brilliant Blue (CBB) | Dark blue | Ground parenchymatous tissue |
| 3 | Lipid | Neutral Red | Red | Cortical parenchyma |
| 4 | Tannin | Ferric Chloride | Brownish black | Cortical parenchyma |
| 5 | Crystal | Polarised light | Bright white | Cortex |
| 6 | Starch | Iodine Potassium Iodide (IKI) | Black or blue | Cortical tissue |
| 7 | Steroid | 2 ml chloroform + Sulphuric acid + Acetic acid | Green colour | Cortex |
| 8 | Phenol | Anhydrous Ferric chloride + 90% Ethanol | Brown to black | Epidermis and Vascular bundle |
| 9 | Flavonoid | 25% lead acetate | Yellow | Epidermis and Vascular bundle |
Table 1. Histochemical localization of secondary metabolites in the tuberous root of Ceropegia juncea Roxb.
Powder microscopic analysis of Ceropegia juncea Roxb. tuberous root
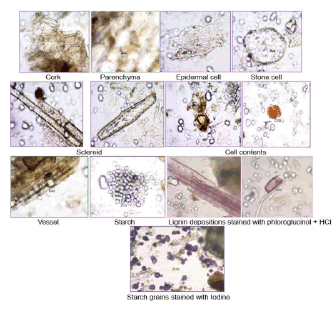
The powder sample is pale buff coloured with characteristic taste and odour; shows the characters like cork cells, parenchyma cells, epidermal cells in surface view. The stone cells, sclereids, lignified reticulate vessels and starch grains were present (Fig. 2).
Figure 2. Powder microscopic analysis of Ceropegia juncea Roxb. tuberous root.
Discussion
The histochemical analysis of Ceropegia bulbosa Var. lushii all tests occasioned in the presence of starch, tannin, mucilage, crystals, crystals, oil, and lignin in leaves, whereas stem showed the absence of starch, mucilage, crystals, oil and the root do not encompass mucilage and crystals (Bhandari, et al. 2016) and the Rauvolfia serpentina root consists of starch, tannin, alkaloids, flavonoids, reducing sugar, protein and amino acids (Das, et al. 2017). The plant’s anatomical and powder microscopic examination identifies a number of diagnostic features. It is evident from the leaf anatomy that both anomocytic and anisocytic stomata are present. Unicellular trichomes exist in the leaf’s polygonal, thin-walled epidermal cells. It is feasible to observe complex cells with oil globules inside the collenchymatous tissue. It is significant that the leaf tissue lacks calcium oxalate crystals. Sclereids are random stone cells that are a characteristic of the stem anatomy and contribute to mechanical support. The leaf’s presence of several vessel features, such as spiral, lignified scleriform, and pitted vessels, were further revealed by powder microscopy (Krishnaiah, et al. 2021). Total lipids, trepenes, acidic and neutral lipids, phenolic compounds, lignin, tanins, pectin, starch, alkaloids, and proteins are all detected by anatomical and histochemical study of Anethum graveolens L., Catharanthus roseus (L) G. Don, and Ruta graveolens L. (Mohammed, et al. 2022).
Conclusion
The Ceropegia juncea Roxb. have a wide range of chemical constituents. Histochemical analyses show the pure identity of phytoconstituents and it is employed to find out the phytomedicine. We must correlate customary acquaintance with contemporary remedy to find new medicines.
Acknowledgements
The author’s Research Scholar Ms. M. Bhuvaneswari (TNSCST/RFRS/05/VM/2021-22) and Research Guide Dr. C. Chitra Vadivu, PG and Research Department of Botany, Vellalar College for Women (Autonomous), Thindal, Erode express heartfelt gratefulness for “Catalyzed and financially supported by Tamil Nadu State Council for Science and Technology, Department of Higher Education, Government of Tamil Nadu” for the project (RFRS)”. We express our gratitude to Central Research Laboratory and PG and Research Department of Botany, Vellalar College for Women (Autonomous), Thindal, Erode for utilizing instrumentation, extend thanks for getting advice from the Department of Pharmacognosy, “Siddha Central Research Institute (CCRS)”, Ministry of Ayush, Govt. of India, Chennai.
References
- Arora S, Meena S. (2018). Anatomical studies on medicinal and endangered CAM plants of Milkweed family from Thar Desert, Rajasthan, India. Ann Plant Sci.7:2115-2120.
- Pearse AGE. (1988). Histochemistry: Theoretical and applied. 1st ed. London: Longman.
- Krishnamurthy KV (1998). Methods in plant histochemistry. Chennai: S. Viswanathan. 90.
- Krishnan S, Ebenenzer GAI, Dayanandan P. (2001). Hostochemical localisation of storage components in caryopsis of rice (Oryza sativa L.). Curr Sci. 80:567-571.
- Krishnan S, Dayanandan P. (2003). Structural and histochemical studies on grain filling in the caryopsis of rice (Oryza sativa L.). J Biosci. 28:455-469.
- Alamgir ANM. (2017). Microscopy in pharmacognosy. In: Alamgir ANM, editor. Therapeutic use of medicinal plants and their extracts. Pharmacognosy. Cham: Springer. 1:497-513.
- Kadam VB, Famila S, Tambe SS, Momin RK (2013). Histochemical investigation of different organs of two medical plants in Maharashtra. Int J Pharm Res Biosci. 2:194-201.
- Singh D, Aeri V, Ananthanarayana DB. (2018). Development of standard operating protocol for slide preparation of powdered bark samples with varying grinding techniques. Pharmacog J. 10.
- Pradeepika C, Selvakumar R, Nabi MSU, Sajeev MS, Giri AN. (2018). Histochemical and phytochemical studies on selected medicinal plants. Pharma Innov J. 7:192-196.
- Karayil S, Bhavani V, Vivek CH. 2014. Heavy metal analysis from traditionally used herb Ceropegia juncea (Roxb.). 4:7-11.
- Harborne JB. (1973). Phytochemical methods. London: Chapman and Hall Ltd. 49-188.
- Kokate CK, Purohit AP, Gokhale SB (1990). Pharmacognosy. 1st ed. Pune: Nirali Prakashan. 150-152.
- Bhandari M, Bhandari A, Bhandari A. (2016). Anatomical, physico-chemical, and phytochemical investigations of Ceropegia bulbosa var. lushii. Indian J Nat Prod Resour. 7:314-322.
- Das AA, Das UD, Nag SN. (2017). Studies on pharmacognosy, micromorphology and histochemical localization of few phytochemicals in medicinal plants in the lateritic belt of West Bengal. Plant Arch. 17:1133-1138.
- Gamble JS (1935). Flora of the Presidency of Madras. Vol. I-III. Calcutta: Botanical Survey of India.
- Krishnaiah D, Devi T, Bano A, Sarbatly R. (2021). Pharmacognostic and phytochemical investigations on Catharanthus pusillus (Murray) G. Don. SSRN. 1:67-72.
- Mohammed MN, Nassrullah IK. (2022). Stem histochemistry analysis of three medicinal species from Iraq. J Gen En Resources Con. 10:219-224.