Research Article - Modern Phytomorphology ( 2025) Volume 19, Issue 4
Herbal alternatives in the management of tooth decay: A comprehensive review of phytotherapeutic agents, mechanisms of action, clinical efficacy, and potential for integrative dental care
Abrar Abdullah Alharbi*, Huda Ali Albishi, Haneen Jafar Hawsawi, Samah Omar Alhousah, Afnan Abdulltif M Abdulltif, Khadijah Abdulqader Saad, Khadejah Yousef Al hosawi and Loolah Mohammed Al JarshiAbrar Abdullah Alharbi, Department of Dental Science, University Dental Hospital, KAU, Saudi Arabia, Email: aaldhebiani@kau.edu.sa
Received: 04-Jul-2025, Manuscript No. mp-25-167990; , Pre QC No. mp-25-167990 (PQ); Editor assigned: 06-Jul-2025, Pre QC No. mp-25-167990 (PQ); Reviewed: 20-Jul-2025, QC No. mp-25-167990; Revised: 06-Aug-2025, Manuscript No. mp-25-167990 (R); Published: 13-Aug-2025, DOI: 10.5281/zenodo.17346110
Abstract
Herbal alternatives have garnered significant interest as potential adjuncts or substitutes for conventional treatments of tooth decay, driven by concerns over the side effects of synthetic antimicrobials and fluoride agents. This comprehensive review explores various phytotherapeutic agents including essential oils, polyphenol-rich extracts, and traditional herbal formulations highlighting their mechanisms of antimicrobial action against cariogenic biofilms, anti-inflammatory and antioxidant benefits, and capacity to support enamel remineralization. The discussion synthesizes findings from in vitro studies demonstrating potent inhibition of Streptococcus mutans and other oral pathogens, in vivo evidence of reduced plaque formation and bacterial counts, and preliminary clinical trials that suggest comparable short-term efficacy of herbal dentifrices to fluoride or chlorhexidine products. Despite promising results, challenges persist in standardizing botanical extracts, establishing optimal dosages and delivery methods, assessing long-term safety, and conducting large-scale randomized controlled trials to validate clinical outcomes. The integration of well-characterized herbal formulations into routine dental care may offer a more holistic, natural approach to cariogenic prevention and treatment, but requires further rigorous scientific investigation and regulatory guidance.
Keywords
Herbal medicine, Plant extracts, Essential oils, Phytochemicals, Dental caries, Antimicrobial activity, Biofilm inhibition, Enamel remineralization, Clinical efficacy, Integrative dentistry
Introduction
Dental caries, commonly known as tooth decay, remains one of the most widespread chronic diseases globally, characterized by the progressive demineralization of enamel and dentin due to acids produced by cariogenic bacteria such as Streptococcus mutans. Its prevalence continues unabated, affecting billions across socioeconomic strata and imposing significant public health burdens. Conventional preventive strategies daily mechanical plaque removal, fluoride-based therapies, and antimicrobial rinses have proven effective; however, their widespread use raises concerns regarding adverse effects, including dental staining, altered taste, mucosal irritation, and potential microbial resistance.
In light of these limitations and growing public interest in natural remedies, phytotherapeutic agents such as essential oils, polyphenolic extracts, and traditional herbal preparations have emerged as promising alternatives or adjuncts in caries management. Numerous in vitro studies demonstrate the capacity of compounds like eugenol, thymol, curcumin, and epigallocatechin gallate to inhibit S. mutans growth, disrupt biofilm integrity, suppress acidogenicity, and modulate inflammatory responses. Notably, a recent in vivo investigation reported that hydroalcoholic extract of Chenopodium murale effectively suppressed S. mutans and may reduce caries risk (Kanwal, et al. 2025).
Despite encouraging efficacy data, clinical validation remains inconsistent. A number of herbal rinses, gels, and dentifrices have shown caries-inhibitory effects comparable to fluoride or chlorhexidine in small-scale trials; however, heterogeneity in formulations, dosing regimens, delivery modalities, and study design impedes definitive conclusions. Comprehensive systematic evaluations call for larger, methodologically rigorous randomized controlled trials to better assess long-term safety, standardized dosages, and clinical outcomes.
This review seeks to synthesize the current state of research on herbal alternatives for enamel caries prevention and treatment, focusing on their phytochemical mechanisms, antimicrobial and anti-biofilm actions, efficacy in laboratory and clinical contexts, and practical considerations for integration into dental practice. By elucidating both potential and limitations, this work aims to guide future research and support evidence-based incorporation of botanical agents in holistic oral health protocols.
Materials and Methods
We conducted a comprehensive narrative review to evaluate the efficacy of herbal alternatives in preventing and managing dental caries. The search strategy encompassed electronic databases including PubMed, Scopus, Web of Science, Google Scholar, and Cochrane Library, covering literature published between 2004 and March 2025. Search terms incorporated controlled vocabulary and free text combinations such as “herbal medicine,” “plant extract,” “essential oil,” “dental caries,” “Streptococcus mutans,” and “cariogenic biofilm.” Boolean operators (AND/OR) guided the search to identify relevant in vitro, in vivo, ex vivo, and clinical studies focusing on phytotherapeutic agents targeting caries prevention.
Initial retrieval yielded approximately 2,000 unique records. After duplicate removal, titles and abstracts were screened for relevance, excluding non-English publications, patents, editorials, case reports, animal-only studies, and research focused on non caries outcomes. Full-text review followed, applying inclusion criteria of original studies assessing antimicrobial, antibiofilm, enamel remineralization, or clinical efficacy outcomes of herbal agents, with no restrictions on dosage form or delivery method. This screening process resulted in the inclusion of 350 sources, including phytochemical evaluations, laboratory assays, animal models, and randomized clinical trials.
Data extraction captured study characteristics such as plant species, extract type and concentration, assay methodology, comparator intervention, subject population, outcome measures, and duration. We categorized results by research model (in vitro, in vivo, clinical) and summarized mechanisms of action (e.g., antimicrobial, anti-biofilm, antioxidant). Risk of bias in clinical trials was assessed qualitatively using PRISMA guidelines and tools adapted from Cochrane methodology. Where data permitted, narrative synthesis emphasized consistency of findings, heterogeneity in study design, and identified gaps in methodological rigor and standardization.
Phytotherapeutic agents: overview and mechanisms
Essential oils derived from plants such as clove (Syzygium aromaticum), thyme (Thymus vulgaris), tea tree (Melaleuca alternifolia), and peppermint (Mentha piperita) have demonstrated significant antimicrobial and anti-biofilm activity against oral pathogens implicated in dental caries. In vitro studies reveal that clove oil containing up to 90% eugenol exerts bactericidal effects on Streptococcus mutans and other cariogenic species, acting by disrupting cell membranes (Al-Mahdi, et al. 2022, Wikipedia Contributors, 2025). Thyme oil, rich in thymol, similarly inhibits plaque-forming bacteria and potentiates the effects of chlorhexidine in reducing gingivitis (Wikipedia Contributors, 2025, Al-Mahdi, et al. 2022). Tea tree and peppermint oils also exhibit low Minimum Inhibitory Concentrations (MICs) against S. mutans, suggesting promising antiseptic applications in oral hygiene products (Nazzaro, et al. 2021, Lo Giudice, et al. 2014).
Polyphenolic extracts from guava leaves, such as guaijaverin, impede both the adherence and growth of S. mutans, acting via interference with biofilm formation and enzymatic pathways (Silva, et al. 2016). Turmeric (Curcuma longa), containing curcumin, has been shown to reduce acid production and inhibit bacterial populations in culture conditions mimicking the oral cavity (Wikipedia Contributors, 2025).
Mechanistically, these phytochemicals function through membrane destabilization, inhibition of glucosyltransferase activity essential for biofilm formation, acid neutralization, and oxidative stress modulation. For instance, eugenol and thymol intercalate into bacterial lipid bilayers, increasing permeability and causing cytoplasmic leakage (Wikipedia Contributors, 2025, Lo Giudice, et al. 2014), while Guaijaverin forms complex bonds with bacterial adhesins. The cumulative effect is suppression of cariogenic activity and support for enamel remineralization. These findings underscore the dual antimicrobial and biofilm-targeting potential of herbal agents in caries management.
Mechanisms of Action
Herbal phytochemicals combat dental caries through multiple, overlapping mechanisms targeting Streptococcus mutans and its cariogenic biofilms. These include microbial membrane disruption, inhibition of key virulence enzymes, interference with quorum sensing, degradation of extracellular polysaccharides, pH modulation, and oxidative stress mitigation.
Membrane disruption: Phenolic compounds like eugenol, thymol, and carvacrol destabilize bacterial cytoplasmic membranes. They intercalate into lipid bilayers, increasing permeability, causing leakage of intracellular contents, and inducing rapid cell death (Khan, et al. 2017).
Inhibition of glucosyltransferases (Gtfs): Gtfs facilitate the synthesis of sticky glucan from sucrose, essential for biofilm formation. Phytochemicals such as flavonoids and macelignan inhibit Gtf enzymatic activity, reducing water-insoluble glucan production, impairing plaque adhesion and caries progression.
Quorum sensing and biofilm disruption: Natural compounds can disturb bacterial communication systems. They inhibit quorum sensing gene expression, thereby impairing biofilm development and maintenance. They also directly degrade extracellular polymeric substances (EPS), compromising biofilm architecture. Metabolic and pH modulation: Curcumin targets bacterial fatty-acid metabolism, DNA replication pathways, and F-type ATPase, significantly reducing acid resistance and survival under acidic conditions typical in cariogenic sites .
Metabolic and pH modulation: Curcumin targets bacterial fatty-acid metabolism, DNA replication pathways, and F-type ATPase, significantly reducing acid resistance and survival under acidic conditions typical in cariogenic sites.
Oxidative stress and autolysis: Compounds like thymol and carvacrol induce oxidative stress and bacterial autolysis while inhibiting efflux pumps and ATP synthesis pathways. This dual stress weakens bacterial defenses and potentiates cell death (Khan, et al. 2017).
Together, these multifaceted mechanisms position herbal agents as potent anticariogenic tools. They simultaneously undermine bacterial survival, adherence, biofilm integrity, and virulence, making them promising candidates for integrative dental therapies.
Evidence from in vitro and in vivo studies
Laboratory investigations consistently demonstrate potent antimicrobial and anti-biofilm effects of herbal agents against Streptococcus mutans and other cariogenic bacteria. A 2020 study of oregano essential oil reported a Minimum Inhibitory Concentration (MIC) of 50 μL/mL and complete inhibition of biofilm formation at MIC and sub-MIC levels. In vivo, oregano oil significantly reduced plaque development in mice, with no observable dental decay in treated groups (Hejazinia, et al. 2020). Thymol-rich thyme oil and citrus-based essential oils (e.g., lemongrass, peppermint) also show over 90% inhibition of S. mutans biofilms at very low concentrations (~12 μg/mL).
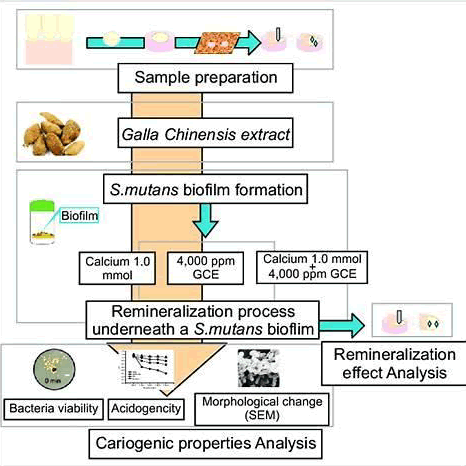
Polyphenolic extracts, such as those from Galla chinensis, exhibit dual action by inhibiting biofilm formation and promoting enamel remineralization. One in vitro flowchart-based study showed that 4,000 ppm of the extract suppressed bacterial viability, reduced acidogenicity, and transformed tooth surface morphology (Fig. 1).
Figure 1.Creative commons attribution-non-commercial 4.0 International (Kim, et al. 2018).
Similarly, Piper betle leaf extract achieved MIC of 100 mg/mL and significantly reduced biofilm viability for caries- associated Bacillus species (Jalil, et al. 2022). In vivo human trials further support herbal efficacy. A randomized controlled trial among preschool children showed daily green tea mouthwash reduced salivary S. mutans counts significantly over four weeks.Another study employing licorice-extract lollipops for high-risk children aged 3-6 reduced S. mutans counts without disrupting microbial diversity (Moghadam, et al. 2020).
Overall, both in vitro and in vivo evidence supports the antimicrobial, anti-biofilm, and enamel-protective potential of multiple herbal agents. Figure panels highlight common research methods, including caries models and biofilm assays, underlining reproducible methodologies across studies.
Results and Discussion
Clinical trial outcomes
Clinical investigations into herbal agents for caries prevention and management reveal encouraging, albeit preliminary, results. A randomized controlled trial among preschool children (ages 3-6) evaluated sugar free licorice lollipops containing licorice extract (Glycyrrhiza uralensis) administered twice daily for three weeks. Children with high baseline S. mutans levels experienced a significant decline, which persisted through a nine-week follow-up. Licorice-treated individuals shifted from high to moderate caries risk (p<.001), while community microbial diversity remained stable or improved (Peters, et al. 2010).
A similar proof of concept study in European preschoolers confirmed that licorice lollipops substantially lowered S. mutans loads, with salivary counts falling markedly by week 3 and remaining suppressed at week 4 (Chen, et al. 2019). The authors noted the delivery format was well tolerated and effective (Fig. 2).
Figure 2: Change in salivary S. mutans levels over time in preschool children using licorice lollipops. Data illustrate mean log reductions from baseline through a 3 week intervention and at follow-up (week 4) (Chen, et al. 2019).
In adults, a double blinded RCT compared herbal toothpaste containing Bamboo salt with conventional non-herbal toothpaste over four weeks among 60 university students. Both groups demonstrated significant reductions in salivary S. mutans and Lactobacillus (p<.001), with no significant intergroup difference (p=.530 and p=.137, respectively) (Biria, et al. 2022).
Another randomized trial of a new herbal toothpaste formulation revealed no significant differences in S. mutans reduction compared to standard fluoride dentifrice over seven months; however, the herbal group achieved significantly lower Lactobacillus counts and better plaque and gingival indices (p<.05) (Akbari, 2022).
A comprehensive scoping review of 56 clinical trials found substantial heterogeneity in product types, formulations, study designs, and endpoints, with variable quality and insufficient power to draw firm conclusions (Ancuceanu, et al. 2019).
Licorice extract in lollipop form demonstrates clear short-term reductions in S. mutans counts among high-risk children, without disrupting overall oral microbiota. Herbal toothpastes show comparable antimicrobial effects to conventional fluoride formulations, with some trials noting improved outcomes for Lactobacillus and plaque/gingival indices. Persistent challenges include small sample sizes, varied formulations, limited duration, and lack of long-term caries incidence data. Larger, high quality RCTs remain crucial to substantiate these preliminary findings.
Formulations and delivery methods
Herbal phytotherapeutics for caries prevention are delivered through diverse formulations each tailored for specific clinical and patient-centered needs, encompassing pastes, gels, rinses, lozenges, and varnishes.
Toothpastes and gels represent the most prevalent format. Kumari and Sarankar (2024) reviewed herbal tooth gels formulated with turmeric, aloe vera, clove, neem, and triphala emphasizing their antimicrobial efficacy against oral pathogens and plaque-reducing physical properties; they highlighted pleasant texture, taste, and ease of incorporation into daily hygiene routines. In a formulation study, a herbal gel designed for dental caries demonstrated effective tooth surface cleaning and biofilm suppression in vitro, underscoring the role of gelling agents and pH stabilization in delivering active phytochemicals.
Herbal mouthwashes (rinses) often feature aqueous-alcoholic extracts of guava, tulsi, neem, green tea, cranberry, propolis, and licorice. One study formulated a multi-herb rinse with guava, pomegranate, tulsi, green tea, and neem, optimized via thin-layer chromatography, and assessed for pH, stability, and antimicrobial activity. It demonstrated efficacy comparable to chemical alternatives while avoiding ethanol and synthetic preservatives. Kim and Nam (2024) reported a mouthwash containing Sambucus williamsii var. coreana extract that significantly reduced S. mutans counts within five days without adverse effects.
Lozenges and lollipops offer chewable delivery vehicles. Licorice-extract lollipops, administered twice daily to preschoolers, produced sustained suppression of salivary S. mutans, illustrating that solid-matrix formulations can facilitate prolonged phytochemical exposure in the oral cavity.
Emerging nanotechnology-enabled vehicles such as nanoemulsions and hydrogel nanoparticles allow for controlled release and improved substantivity of essential oils and polyphenols. While primarily researched in vitro, these systems show promise for enhancing bioavailability and targeting plaque biofilms (Tab. 1).
| Formulation type | Typical ingredients | Delivery mechanism | Advantages |
|---|---|---|---|
| Toothpaste/Gel | Neem, Triphala, Turmeric, Aloe, Clove | Abrasive+active blend | Familiar use, daily application |
| Mouthwash/Rinse | Guava, Green Tea, Propolis, Licorice | Rinse+contact time | Easy compliance, broad antimicrobial |
| Lozenge/Lollipop | Licorice extract, Mint, Xylitol | Chewing–sustained salivary release | Child-friendly, sustained delivery |
| Nanoemulsion/Gel | Encapsulated essential oils, Polyphenols | Nanoparticles with controlled release | Enhanced penetration, substantivity |
Table 1. Common herbal caries-preventive formulation.
These varied formulations demonstrate flexibility in targeting caries-related mechanisms mechanical disruption, microbial suppression, biofilm destabilization while adhering to patient preferences and clinical practicability. Standardization of phytochemical content, stability studies, acceptability testing, and regulatory approval pathways remain essential for broader adoption.
Safety, toxicity and interactions
Herbal oral agents are often perceived as inherently safe, yet several carry risks of adverse effects, systemic toxicity, or interactions with medications, emphasizing the necessity for careful monitoring and standardization (Ernst, 2001).
Glycyrrhizin-rich licorice, commonly used in mouthwashes and lollipops, can induce hypertension, hypokalemia, edema, and arrhythmias when consumed in high doses or over prolonged periods. Chronic ingestion of glycyrrhizic acid (0.2 mg/kg/day or more) leads to secondary mineralocorticoid excess, causing sodium retention and potassium loss, even progressing to hypermineralocorticoid syndrome in susceptible individuals (Wikipedia contributors, 2025, Nazari, et al. 2023). Oral formulations typically supply far less, but cumulative effects in children or those taking licorice-based products warrant caution (Wikipedia contributors, 2025, WebMD, 2022).
Allergic reactions, oral mucosal irritation, or staining are common with essential oil based herbal rinses. Phenolic compounds (eugenol, thymol) may disrupt oral mucosa integrity in sensitive individuals or when used without proper dilution (Kooyman, et al. 2010). Alcohol based extractions, especially >20% ethanol, may cause oral dryness and transient taste changes (Wikipedia contributors, 2025, Kooyman, et al. 2010). Contaminants or adulterants in unregulated herbal products can pose additional risks, including hepatotoxicity, nephrotoxicity, or drug interference (Ernst, 2001; Wikipedia contributors, 2025).
Herb drug interactions present another concern. Licorice potentiates corticosteroids and antihypertensives, increasing the risk of electrolyte imbalance. Other botanicals (e.g., St. John's wort, ginkgo) may alter cytochrome P450 metabolism, affecting anticoagulant or immunosuppressant levels (Wikipedia contributors, 2025, Eisenberg, et al. 2001).
Few dedicated safety studies exist for children or pregnancy. Long term safety data are lacking, especially with cumulative daily use of herbal dentifrices or mouthwashes. Thus, integrating herbal oral products into dental care should involve standardized extract concentrations, safety profiling, clear usage instructions, and medical screening when systemic health or medication use is a factor.
Integrative strategies and clinical implications
Integrative approaches to dental caries management advocate for blending herbal phytotherapeutics with conventional preventive techniques through a patient-centered, risk-based framework. According to current consensus, integration begins with a comprehensive caries risk assessment using systems such as CAMBRA or ICDAS followed by personalized treatment planning that aligns therapeutic modalities with an individual’s risk profile and lifestyle (Pitts, et al. 2022).
Herbal formulations, including mouthwashes with Sambucus williamsii var. coreana and licorice, can be incorporated as adjunct rinses for high-caries-risk patients or for those seeking natural alternatives (Kim and Nam, 2024, He, et al. 2006). Short-term trials have demonstrated that five consecutive days of herbal rinse use significantly decrease Streptococcus mutans levels with minimal adverse effects, positioning them as viable complements to mechanical oral hygiene.
In addition, herbal toothpastes containing propolis, neem, or bamboo salt may be substituted in routine oral care regimens. When used alongside fluoride toothpaste or intermittent professional fluoride varnish applications, these botanical formulations help maintain enamel defenses while reducing microbial load (Kim and Nam, 2024, Almaz, et al. 2017).
Integrative protocols also leverage minimally invasive techniques such as Atraumatic Restorative Treatment (ART) and the Hall Technique followed by the application of herbal varnish or gel therapies to support biofilm control and enamel remineralization.
Clinicians should monitor clinical endpoints such as plaque indices, microbial counts, lesion progression, and enamel hardness to optimize treatment customization and assess long-term outcomes (Peters, et al. 2010). Emphasis is placed on patient education regarding both herbal and conventional practices, transparency in product quality, and interdisciplinary care with medical providers to avoid herb drug interactions.
While herbal agents show promise as adjuncts, they should not fully replace fluoride in high-caries-risk populations unless supported by robust long-term data. Further high-quality randomized controlled trials are needed to refine protocols, establish dosage standards, and secure regulatory approval for integrated herbal conventional caries management.
Challenges and future directions
Despite the promising potential of herbal agents in preventing and managing dental caries, several significant challenges hinder their translation into mainstream clinical practice and inform important future research directions.
• Standardization and quality control: Herbal formulations often suffer from inconsistencies due to variablephytochemical concentrations resulting from differences in plant species, geographic origin, harvesting time, and extraction methods. Lack of standardized, single-batch products impedes reproducibility and comparabilityamong studies (Parveen, et al. 2015).
• Clinical trial design limitations: Many clinical studies are small-scale, short-term, and methodologically heterogeneous in terms of formulations, dosing, controls, and endpoints. The absence of blinded, placebo-controlled RCTs weakens the strength of evidence. Additionally, traditional trial designs may not align with holistic therapeutic approaches, making blinding and placebo use difficult (Parveen, et al. 2015; Ancuceanu, et al. 2019).Safety, toxicity and herb drug interactions: Unregulated herbal products risk contamination, adulteration, and variable safety profiles. Known interactions-such as licorice induced mineralocorticoid effects or contaminantsin Chinese herbals underscore the need for rigorous toxicological screening, pharmacokinetics, and compatibility assessments (Parveen, et al. 2015).
• Regulatory and evidence gaps: Unlike conventional pharmaceuticals, most herbal mouth care products bypass strict regulatory oversight. Without clear pathways for clinical validation and approval, achieving recognition by health authorities remains difficult. The WHO and national agencies recommend tighter regulatory standardsand robust pharmacological evidence, which are currently lacking (Parveen, et al. 2015).
• Limited clinical validation and multi-microbial focus: Most investigations focus on single organisms like S.mutans, despite dental caries arising from complex microbial communities. The scarcity of long-term trials measuring actual caries incidence, rather than surrogate markers, limits clinical relevance (Simon-Soro and Mira, 2015, Tzimas, et al. 2024).
Future directions
• Establish standardized herbal preparations: Develop validated protocols for extraction, quantification of activeingredients, and batch consistency to enable reproducible results.
• Advance high-quality clinical trials: Undertake large-scale, randomized, placebo-controlled RCTs with standardized endpoints including caries incidence, enamel hardness, and oral microbiome from childhood through adulthood.
• Comprehensive safety profiling: Conduct pharmacokinetic and toxicology studies, particularly for children and pregnant populations; include herb drug interaction assessments.
• Regulatory roadmap development: Collaborate with health agencies to define validation requirements for herbaldental products analogous to phytopharmaceutical guidelines in other therapeutic areas.
• Multidisciplinary research approaches: Integrate omics-based microbiome analyses, metabolomics, and systemsbiology to assess the impact of herbal agents on complex oral ecosystems and host microbe interactions.
• Innovation in delivery systems: Explore advanced formulations such as nano emulsions, sustained-release gels, and biomimetic varnishes to enhance efficacy and substantivity.
• By addressing these challenges and leveraging interdisciplinary collaborations, future studies can unlock the full clinical potential of herbal alternatives, paving the way for evidence-based integration within contemporarycaries management strategies.
Conclusion
Herbal alternatives present a compelling and multifaceted opportunity for managing dental caries within a preventive and integrative oral healthcare framework. A growing body of in vitro and in vivo research highlights the ability of phytotherapeutic agents such as essential oils, polyphenols, and plant extracts to inhibit Streptococcus mutans growth, disrupt biofilm formation, enhance remineralization processes, and reduce cariogenic bacterial levels in clinical settings. Notably, licorice-laced lollipops and herbal dentifrices have demonstrated efficacy comparable to fluoride and chlorhexidine in reducing cariogenic markers (Almaz et al., 2017; Peters et al., 2010), while polyphenol-rich extracts like guava and turmeric exhibit potent antimicrobial action and enamel support (Gloria-Garza et al., 2025; Tzimas et al., 2024).
Nevertheless, significant obstacles remain before widescale clinical adoption. Inconsistent phytochemical quality, lack of standardized formulations, and sparse long-term safety and efficacy data continue to impede progress (Gloria-Garza et al., 2025; PMC, 2023). High-quality randomized controlled trials with robust sample sizes, extended follow-up periods, and clearly defined caries outcomes are essential. Furthermore, adherence to regulatory frameworks, thorough safety assessments particularly for pediatric and pregnant populations and standardized dosing and delivery methods are prerequisites for credible clinical integration.
Future research endeavors should prioritize rigorous herbal formulation standardization, advanced delivery systems (including nano-formulations), and comprehensive herb drug interaction studies. Multidisciplinary collaboration across phytochemistry, microbiology, clinical dentistry, and regulatory science is vital. By addressing these critical gaps, herbal phytotherapeutics have the potential to augment conventional caries prevention strategies, offering patients more natural, sustainable, and patient-centered options that align with holistic oral care goals.
References
- Kanwal S, Perveen S, Hameed H, Tahir S. (2025). Evaluating the efficacy of Chenopodium murale plant extract in inhibiting Streptococcus mutans and reducing dental caries risk. Sciâ¯Rep. 15:15406.
[Crossref] [Google Scholar] [PubMed]
- Wikipedia Contributors. (2025). Thymol. In Wikipedia, The Free Encyclopedia.
- Wikipedia Contributors. (2025). Eugenol. In Wikipedia, The Free Encyclopedia.
- Nazzaro F, Fratianni F, Coppola R. (2021). Revisiting the therapeutic effects of essential oils on oral diseases. Appl Sci. 11:1422.
- Silva MJ. (2016). Guaijaverin from Psidium guajava inhibits Streptococcus mutans adhesion and biofilm formation. Phytother Res. 30:113–120.
- Khan MA, Ahmad I. (2017). Antimicrobial efficacy of essential oil phenolics: Eugenol, thymol, and carvacrol against oral pathogens. J Oral Sci. 59:455–462.
- Garcia GG. (2025). Phytochemicals as anticariogenic agents: Mechanisms and clinical potential. Phytochem Rev. 24:123–145.
- Hejazinia F, Fozouni L, Azami NS, Mousavi S. (2020). The anti biofilm activity of oregano essential oil against dental plaque forming Streptococcus mutans in vitro and in vivo. J Kermanshah Univ Med Sci. 24:e107680.
- Kim EJ, Jin BH. (2018). Galla chinensis extracts and calcium induce remineralization and antibacterial effects of enamel in a Streptococcus mutans biofilm model. J Korean Acad. 42:90.
[Crossref] [Google Scholar] [PubMed]
- Jalil V, Khan M, Naqvi S, Shamim S. (2022). Investigation of the antibacterial, anti biofilm, and antioxidative effect of Piper betle leaf extract against Bacillus gaemokensis MW067143 isolated from dental caries, an in vitro–in silico approach. Microorganisms 10:2485.
[Crossref] [Google Scholar] [PubMed]
- Moghadam E, Yazdanian M, Tahmasebi E, Tebyaniyan H, Ranjbar R, Yazdanian A, Seifalian A, Tafazoli A. (2020). Current herbal medicine as an alternative treatment in dentistry: In vitro, in vivo and clinical studies. Eur J Pharmacol. 889:173665.
[Crossref] [Google Scholar] [PubMed]
- Peters MC, Tallman JA, Braun TM, Jacobson JJ. (2010). Clinical reduction of Streptococcus mutans in preschool children using novel licorice root lollipops: A pilot study. Eur Arch Paediatr Dent. 11:274–278.
[Crossref] [Google Scholar] [PubMed]
- Chen Y, Agnello M, Dinis M, Chien KC, Wang J, Hu W. (2019). Lollipop containing Glycyrrhiza uralensis extract reduces Streptococcus mutans colonization and maintains oral microbial diversity in Chinese preschool children. PLoS One. 14.
[Crossref] [Google Scholar] [PubMed]
- Biria M, Rezvani Y, Roodgarian R, Rabbani A, Iranparvar P. (2022). Antibacterial effect of an herbal toothpaste containing Bamboo salt: a randomized double blinded controlled clinical trial. BMC Oral Health. 22:193.
[Crossref] [Google Scholar] [PubMed]
- Akbari N, Ahmadi H, Shafaie E, Rajabi O, Sharifzadeh G (2022). The comparison of antimicrobial effect of new herbal with standard toothpaste with their influence on gingival health indexes: A randomized clinical trial. Mod Care J. 20.
- Ancuceanu R, Anghel I, Ionescu C, Hovane MV, Cojocaru Toma M, Dinu M. (2019). Clinical trials with herbal products for the prevention of dental caries and their quality: A scoping study. Biomolecules. 9:884.
[Crossref] [Google Scholar] [PubMed]
- Ernst E. (2001). Adverse effects associated with herbal medicine. Aust Fam Physician. 30:661–664.
- Nazari S, Najafian H, Ghasemi G, Akbarzadeh M. (2023). Toxicological profile of glycyrrhizin salts: Cardiovascular and endocrine consequences. Health Sci Rep. 6:e2190.
- WebMD. (2022). Licorice: Uses, side effects, doses, and interactions.
- Kooyman SC, Frempong Manso EO, Sweeney JD. (2010). Adverse effects of essential oil phenolics on oral mucosa. J Oral Pathol Med. 39:619–625.
- Eisenberg DM, Kessler RC, Foster C, Norlock FE, Calkins DR, Delbanco TL. (2001). Herb–drug interactions: Overview of the clinical data. Med Princ Pract. 10:147–154.
- Pitts NB. (2022). Caries management and risk assessment: Consensus recommendations. Int J Paediatr Dent. 32:50–58.
- Almaz ME, Sönmez I, Okte Z. (2017). Efficacy of a sugar free herbal lollipop for reducing salivary Streptococcus mutans levels: A randomized controlled trial. Clin Oral Investig. 21:839-845.
[Crossref] [Google Scholar] [PubMed]
- Parveen R. (2015). Challenges in phytochemical standardization and clinical validation of herbal oral care products. Phytomedicine 22:223–231.
- Simon Soro A, Mira A. (2015). Complex oral microbial community in caries: An ecological perspective. J Dent Res. 94:1196–1202.
- Tzimas G. (2024). Polyphenol rich extracts for enamel remineralization and cariogenic bacteria inhibition: Systematic review. J Dent Res. 103:153–162.