Research - Modern Phytomorphology ( 2025) Volume 19, Issue 1
Exploring the functional potential of quinoa-wheat UFB against CCl4 induced hepatotoxicity
Kainat Ashfaq, Shinawar Waseem Ali*, Maufia Shafiq and Qurban AliPakistan Council of Scientific and Industrial Research,Laboratories Complex, Lahore, Pakistan
1Department of Plant Breeding and Genetics, Faculty of Agricultural Sciences, University of the Punjab Lahore, Pakistan
2Centre for Applied Molecular Biology, Faculty of Life Sciences, University of the Punjab, Lahore, Pakistan
Department of Horticulture, Faculty of Agricultural Sciences, University of the Punjab Lahore, Pakistan
Shinawar Waseem Ali, Department of Food Sciences, Faculty of Agricultural Sciences, University of the Punjab, Lahore, Pakistan, Email: shinawar.foodsciences@pu.edu.pk
Received: 20-Dec-2024, Manuscript No. mp-24-155977; Accepted: 20-Jan-2025, Pre QC No. mp-24-155977 (PQ); Editor assigned: 23-Dec-2024, Pre QC No. mp-24-155977 (PQ); Reviewed: 08-Jan-2025, QC No. mp-24-155977 (Q); Revised: 14-Jan-2025, Manuscript No. mp-24-155977 (R); Published: 26-Jan-2025, DOI: 10.5281/zenodo.16744205
Abstract
Alterations in lifestyle and dietary habits due to urbanization lead us towards communities facing wide-spread diet related health issues specifically protein-energy malnutrition. Unleavened flatbread (UFB) is a staple food in Asian countries which also serves as a cheap source of protein and energy. This study is intended to utilize quinoa and wheat composite flour for the production of functional unleavened flat bread (UFB) and evaluate its effectiveness to mitigate hepatotoxicity and nephrotoxicity using animal model. Fortification of quinoa in whole wheat flour created a remarkable effect on the nutritional profile, as a significant increase in protein and mineral contents of composite flour blends was observed. In-vivo analyses of functional unleavened flatbread from T3 (75:25%) in Swiss albino male rats showed significant positive results in-case of CCl4 induced hepato- toxicity and nephrotoxicity when compared with unleavened flatbread made from T0. Liver enzymes AST, ALP and total bilirubin were decreased up to 55.5%, 38.38% and 16.67% in UFBT3 treated rats. Reduction in urea (25%), creatinine (42.10%) and uric acid (3.26%) was also observed. Results indicate the perks of quinoa fortification in whole wheat flour which enlighten the potential of this product (UFB) as a functional food. As chapatti (UFB) is the prime course in our local eatery, this study 1st time reveals the potential of Quinoa-Wheat UFB to reduce malnutrition and hepatotoxicity in Pakistan.
Keywords
Composite flour, Chapatti, Malnutrition, Hepatotoxicity, Nephrotoxicity, Designer foods
Introduction
Cereals are serving mankind for ages through their grains and several other types of foods made by these grains. Wheat and rice are located on top in the umbrella of staple foods because they are the best choice for bread production (Belitz and Grosch et al. 2009). Different epidemiological studies prove that, cereals can said to have therapeutic gains too. So, one cannot deny their supremacy to cater health benefits. Different cereals and legumes have been shown to lower blood cholesterol, manage coronary artery diseases, cure colon issues, directing diabetes to a curative track etc. The most admired cereal products in market include bread, cookies/biscuits, noodles, extruded snacks, breakfast cereals, pasta, noodles and cakes among others. Nowadays, people suffering from diabetes, obesity, celiac disease and many other metabolic syndromes are increasing vigorously thus raising the demands and challenges for food industries to manufacture designer foods by exploiting novel food ingredients and latest processing techniques (Tacer-Caba et al., 2015; Ullah et al., 2023; Junaid and Gokce, 2024).
Among the above mentioned grains, quinoa is said to be a new generation of plant for traditional cuisine. Quinoa possesses supreme quality nutritional profile and beneficial for various diseases like celiac disease due to its gluten free character, also its contribution cannot be ignored to treat issues like malnutrition and anaemia. Quinoa is said to be a well-balanced crop in terms of nutrition owing to its high levels of protein composition and essential amino acids content.
Quinoa flour is esteemed as a super food due to its higher protein content (more balanced amino acid composition) in comparison to other cereal flours. Quinoa flour have 16.4% protein, 3.38% fiber, 2.4% fat and 65.82% carbohydrates in it (Jan and Panesar et al., 2018). Quinoa Flour have strong vitamin (Riboflavin, Vit. E & Vit. C), antioxidant and mineral (potassium, zinc, calcium, iron and magnesium) profile (Arneja and Tanwar et al., 2015; Conte and Fadda et al., 2019).
The most important staple food in sub-continent is wheat based chapatti, as wheat is the renowned staple crop and people have been consuming it forages which clearly relates to the satiety level and taste satisfaction (Rasool et al., 2005; Haider et al., 2023; Javed et al., 2024). But, only consumption of wheat based chapatti, contributes to major nutritional issues like malnutrition as wheat flour limits not only lysine in grain form but it lost almost 10% while baking. Also, it possesses low levels of methionine, threonine and tryptophan as diseases like Kwashiorkor and Marasmus are widespread due to protein deficiency (Saab and Rao et al., 1981).
Different studies (Pasko et al., 2010) suggest that bioactive compounds present in quinoa have potential not only to prevent oxidative stress but also helps in reduction of multiple chronic diseases risks as anti-carcinogenic, immune-modulatory, and anti-inflammatory ultimately change the antioxidant status in the organisms (Fuentes and Paredes-Gónzalez, 2013). Toxin-induced liver damage through Carbon Tetrachloride (CCl4) is extensively used in animal models. Liver necrosis, liver cirrhosis and fatty liver are the most notable pathological characteristics of CCl4- induced hepatotoxicity (Khurelbat and Purevkhuu et al,. 2014).
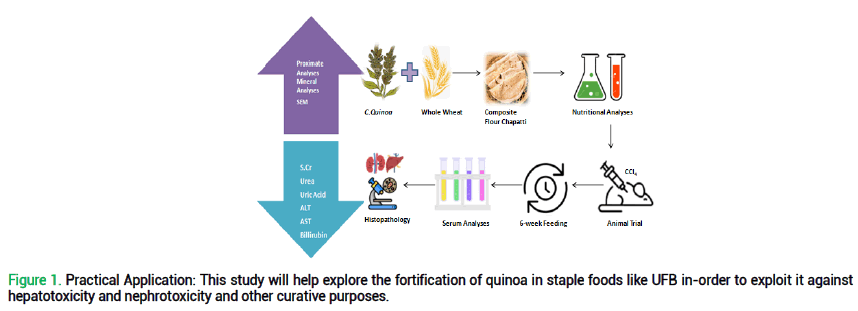
Considering all these concerns, this research is conducted to fill above mentioned gaps by exploring the effect of quinoa-wheat composite flour unleavened flat bread on malnutrition and to assess the effect of quinoa-wheat unleavened flat bread against CCl4 induced liver damage in Swiss albino rats (Fig. 1). This study actually presents a pioneered approach through its contribution in functional foods which significantly considers the staple food of this region yet highlights its therapeutic and nutritional gains. This research not only deals with most prevalent protein energy malnutrition but also serves a functional therapeutic yet, traditional food.
Figure 1. Practical Application: This study will help explore the fortification of quinoa in staple foods like UFB in-order to exploit it against hepatotoxicity and nephrotoxicity and other curative purposes.
More precisely, it’s a groundbreaking study which emphasizes the ameliorative gains of quinoa-wheat composite flat bread against liver and renal damage in a rat model, highlighting the key takeaways in potential health gains of fortifying quinoa into conventional foods. Findings from this research can be meaningfully applied in malnourished areas with prevalence of liver disorders. It’s a nutrient dense therapeutic approach against traditional wheat chapatti which is a fitting resolution to improve diet quality and well-being enhancement.
This investigation distinctively fills the gaps in previously provided literature by highlighting the under-utilization of quinoa in core or primary foods, but also termed quinoa wheat chapatti a light in cave for protein-energy malnutrition as well as a therapeutic diet against liver and kidney damage. Functional foods normally ignore cultural relevance, this functional food is focused on staple food with nutritional and therapeutic gains.
Methodology
Preparation of Quinoa-Wheat Unleavened Flat Bread
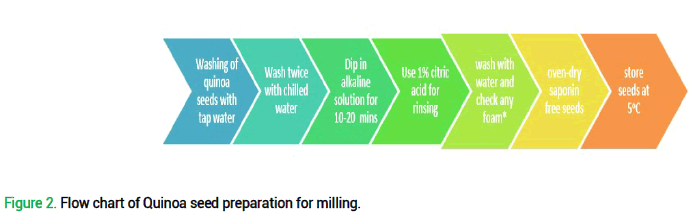
Saponin removal from Quinoa seeds: Before incorporation of quinoa in wheat flour chapatti, saponin removal is mandatory. Saponin removal from quinoa is needed before flour preparation. After two times washing of whole seeds with chilled water, they were dipped in alkaline solution for 10 mins-20 mins. Then 1% citric acid solution was used for rinsing. The unsoiled seeds were purified with water to check any foam to fetch the indication of saponin. Oven-drying of saponin-free seeds was done. After milling of seeds, they were stored at 50C for further analysis (Vilche et al., 2003) (Fig. 2 and Tab.1).
Figure 2. Flow chart of Quinoa seed preparation for milling.
Proximate analyses of composite flours
The analysis of ash, crude fiber, crude protein, and crude fat was carried out as described in AOAC (Baur and Ensminger, 1977).
Mineral analyses of composite flour
For mineral analyses method given by (Horwitz, 1975) was followed. According to this method, weigh the samples carefully and let them subjected to dry ashing. Then digestion of samples with HCL will be done and Zn, Mg, Mn, Cu and Fe were analyzed by atomic absorption spectrophotometer. However, samples which need to be investigated for Ca, Na and K were subjected to wet digestion with HNO3; they were analyzed by flame photometry.
Preparation of chapatti
Chapattis were prepared from quinoa and wheat flour blends as mentioned above. Unleavened-flat bread formulations using wheat and quinoa blends in different ratios are given in tab. 1. Recipe was taken from (Yaseen et al., 2007). Wheat flour blends with 15%, 20%, 25%, and 30% of quinoa flour were used for making unleavened flat bread. Dry material was homogenized (1 gm salt and then 55 ml ± 5 ml water was be added according to water absorption capacity). Dough was manually prepared by kneading for 15 minutes. After that, dough was kept at (25ºC ± 20ºC, 85% RH) for 30 min for proofing. 100g of dough was taken for making bread with roller pin to gain 20cm diameter. Then chapattis were baked at 450 oF at stove for 1 min-2 min.
| Treatments | Formulation of functional Unleavened Flat bread | |||
|---|---|---|---|---|
| Whole Wheat Flour | Quinoa Flour | Salt | Water | |
| T0 | 100 g | 0 g | 1 g | 55 g ± 5 g |
| T1 | 85 g | 15 g | 1 g | 55 g ± 5 g |
| T2 | 80 g | 20 g | 1 g | 55 g ± 5 g |
| T3 | 75 g | 25 g | 1 g | 55 g ± 5 g |
| T4 | 70 g | 30 g | 1 g | 55 g ± 5 g |
Table 1. Flow chart of dough and Unleavened Flat-Bread preparation.
Sensory analysis
After cooling the chapatti to room temperature, their quality characteristics were determined. For this purpose, sensory evaluation was carried out by 10 panellists who were who were highly trained to identify any delicate variation among samples and were initially screened through successive visual and mental acuity tests. Selected panelists were then subjected to multiple trainings to mature their evaluation criteria and to familiarize them to distinct traits of food as flavor, texture, color, crust characters etc. Chapattis were arbitrarily offered to each panelist. Target set for the panelist was to evaluate respective chapatti in-terms of mouth feel, flavor, crumb texture, crust color, crust texture and flavor through nine point scale referring to (Ihekoronye and Ngoddy, 1985) with 9-1: dislike extremely (1); dislike very much (2); dislike moderately (3); dislike slightly (4); neither like nor dislike (5); like slightly (6); like moderately (7); like very much; (8) like extremely (9).
Evaluation was executed in a distraction free controlled environment with consistent lighting. To sustain unbiasedness, panelists were seated individually and any communication among them was strictly avoided. According to standards they were provided with water and crackers to refresh their taste buds between different samples. Provided data was then statistically analyzed with mean scores against each sensory attribute (Lawless and Heyman, 2010).
SEM
Scanning Electron Microscope was used to observe the microstructure of chapatti by the methods given by Prabhasankar et al., (2003) with some modifications.
Experimental design (In-vivo analysis)
Based upon quality and sensory analyses (discussed above), best treatment was chosen for in-vivo studies. For in-vivo trials, male Swiss albino rats were involved. Reasons behind choosing rats as animal model were their physiological similarity to humans most precisely in nutrient metabolism and liver functions, by allowing genetically identical rats in the study make sure the reproducibility of results, they save time of experiment owing to their fast metabolic rates and ultimately quick response to dietary changes and exposure to toxins (Everds and Ramaiah., 2018). 40 male rats were procured and eight groups of five animals in two categories were made according to plan mentioned in tab. 2. Animals for experimentation purpose were retained in cages of stainless steel at a room temperature of 22ºC ± 3ºC with 12h dark/light cycle according to following scheme for 6 weeks. Constituted animal groups were nourished with control sample and the best chosen sample (UFB T3) after sensory analyses adopting the method of Moraes et al., (2012) after little modifications. The CCl4 was injected intraperitoneally via syringe. Rats were then slaughtered for biochemical analysis and liver and kidneys were removed for detailed study.
| Category 1 | Category 2 | Category 3 |
|---|---|---|
| (CCl4 induced Hepatotoxicity) | (CCl4 induced Nephrotoxicity) | (Streptozotocin induced diabetes) |
| Group 1 | Group 1 | Group 1 |
| (Normal control) | (Normal control) | (Normal control) |
| Group 2 | Group 2 | Group 2 |
| (Toxic Control) | (Toxic Control) | (Toxic Control) |
| Group 3 | Group 3 | Group 3 |
| (Toxic Control treated with UFBT3) | (Toxic Control treated with UFBT3) | (Toxic Control treated with UFBT3) |
| Group 4 | Group 4 | Group 4 |
| (Toxic Control treated with UFBT0) | (Toxic Control treated with UFBT0) | (Toxic Control treated with UFBT0) |
Table 2. In-vivo analyses plan of final product.
Efficacy study for CCl4 induced hepatotoxicity
To induce hepatotoxicity in rats, CCl4 was injected intraperitoneally as a toxicant by using a syringe. Subsequently, rats were euthanized and their blood was taken for biochemical analysis Alanine Transaminase (ALT), Aspartate Aminotransferase (AST), Total Billirubin (TB), Serum Urea, Creatinine, Uric Acid, Triglycerides (TG) and Total Cholesterol (TC). CCl4 not only intoxicate liver but have a noticeable toxic effect on kidneys too. Additionally, they were slaughtered to obtain liver, kidneys and pancreas for histopathological examination.
Liver and kidney indices
Alkaline Phosphatase (ALP), Aspartate Aminotransferase (AST) and Total Billirubin (TB) of the collected blood samples was evaluated by the instructions of the supplier with the help of Human kits (Germany). Fawcett and Scott (1960) methods were applied to determine the serum urea, creatinine and uric acid by using kit (Human Germany).
Preparation of tissue homogenate
Tissue homogenate 10% (w/v) was prepared from heart, liver and kidneys in 10 mM phosphate buffer (pH 7.4) using a Potter Elvenjem glass homogenizer (Belco Glass Inc., Vineland, NJ, USA). The homogenate was centrifuged (13000 rpm) for 10 minutes at 4°C. The homogenate was stored in refrigerator until further analysis.
Histopathological examination
Rats were dissected and their organs such as liver and kidneys were preserved in 10% neutral buffered formalin. For histopathological examination, tissues were processed by sequential unit operation including cleaning, fixation, sectioning and staining. After preparation of tissue blocks, 0.4 μm thickness tissues were made using microtome. Hematoxylin and eosin staining was performed following the instructions described by the Bancroft and Gamble (2008).
Statistical analysis
Statistic 8.1 software was employed for statistical analysis of all the performed tests. The significance level was observed utilizing the Least Significant Difference (LSD) test. A one-way analysis of variance was used to analyze the data (Johnson and Bhattacharyya, 2019).
Results
Nutritional profile of quinoa-wheat composite flours
The nutritional profile of the composite flour is known to enhance when whole-wheat flour is fortified with quinoa flour. Tab. 3 shows the nutritional contents of composite flour that has had whole wheat flour displaced with quinoa flour in different treatments (15%, 20%, 25% and 30%). By gradually increasing the amount of quinoa flour in the treatments, the protein content of the composite flour significantly (P ≤ 0.001) rose from 10.14% ± 0.06% in T0 to 11.55% ± 0.04% in T4.
| Treatment | Moisture (%) | Ash (%) | Crude Fibre (%) | Crude Fat (%) | Crude Protein (%) | Carbohydrate (%) |
|---|---|---|---|---|---|---|
| T0 | 13.50 ± 0.52a | 0.56 ± 0.09e | 0.22 ± 0.03c | 1.11 ± 0.03e | 10.14 ± 0.06e | 71 ± 0.09a |
| T1 | 12.98 ± 0.09ab | 1.05 ± 0.03d | 0.88 ± 0.04b | 1.54 ± 0.06d | 10.75 ± 0.04d | 68 ± 0.1b |
| T2 | 12.80 ± 0.1b | 1.23 ± 0.06c | 1.01 ± 0.02a | 1.73 ± 0.04c | 11.02 ± 0.04c | 66 ± 0.06c |
| T3 | 12.62 ± 0.01b | 1.43 ± 0.05b | 1.03 ± 0.05a | 1.95 ± 0.05b | 11.30 ± 0.02b | 63 ± 0.01d |
| T4 | 12.47 ± 0.08b | 1.60 ± 0.02a | 1.05 ± 0.03e | 2.12 ± 0.06a | 11.55 ± 0.04a | 62 ± 0.07e |
| p-value | 0 | 0 | 0 | 0 | 0 | 0 |
Table 3. In-vivo analyses plan of final product.
Studies state that quinoa incorporation provides a protein boost to the overall nutritional profile of the product. This quality of quinoa shows a potential to mitigate the prevalence of protein energy malnutrition. And it’s a better solution to fortify the nutritional profile of unleavened flat breads (Montemurro, Pontonio et al. 2019). Researches also states that in addition of protein enhancement quinoa incorporation in diets can leads to increased crude fat, dietary fibre, essential amino acids and B-vitamins, and this broadens its application in multiple target oriented designer foods (Ogungbenle 2003).
The composite flour samples also had more fat, ash, and crude fiber than the control. Although composite flour contains more protein, ash, and fats than wheat bread, the overall carbohydrate level in composite flour was lower than the control. Composite flour samples exhibit higher levels of ash, dietary fiber, fat, micronutrients and crude fiber when compared with the control sample which clearly depicts quinoa’s supremacy. Its fortification in whole wheat flour leads towards a more balanced diet making sure the availability of essential nutrients which are generally absent in wheat-based meals (Wu 2015). The moisture content was lower in T4 compared to T0 which indicates the water activity is low in quinoa flour as compared to whole wheat flour so increase in quinoa concentration leads to a decrease in moisture content which helps extend the shelf life by controlling microbial spoilage (Jan and Panesar et al. 2019).
Mineral composition of composite flours
Tab. 4 shows the mineral composition of flour treatments with various quinoa flour percentages. There were noticeable changes in the amount of minerals between the composite flour treatments and the control. When quinoa flour levels were increased from 15 to 30% in comparison to the control (100% whole wheat flour), it was discovered that the majority of minerals content steadily increased. Populations effecting from mineral deficiencies can incorporate quinoa in their diet to overcome these issues because its clearly understood from the results that quinoa flour increase in samples leads to a steady increase in mineral profile of the composite flour.
| Treatment | Ca (ppm) | Zn (ppm) | Mg (ppm) | Na (ppm) | Mn (ppm) | K (ppm) | Cu (ppm) | Fe (ppm) |
|---|---|---|---|---|---|---|---|---|
| T0 | 272 ± 0.13e | 14 ±0.04e | 1046 ± 0.01e | 58.40 ± 0.2c | 40.56 ± 0.04a | 2796 ± 0.01a | 3.2 ± 0.05x | 26.15 ± 0.01e |
| T1 | 455.47 ± 0.05d | 16.99 ± 0.08d | 1220 ± 0.22d | 60.21 ± 0.05b | 38.28 ± 0.01b | 1799.18 ± 0.03b | 21.23 ± 0.01b | 35.24 ± 0.02b |
| T2 | 472.14 ± 0.1c | 18.88 ± 0.02c | 1268 ± 0.09c | 60.33 ± 0.02ab | 38.09 ± 0.02c | 1768.59 ± 0.06c | 20.98 ± 0.05c | 29.53 ± 0.1c |
| T3 | 496.23 ± 0.03b | 19.25 ± 0.05b | 1328 ± 0.04a | 60.42 ± 0.04ab | 37.98 ± 0.03d | 1769.44 ± 0.05d | 21.67 ± 0.02a | 76.70 ± 0.03a |
| T4 | 511.64 ± 0.04a | 20.11 ± 0.01a | 1283 ± 0.02b | 60.51 ± 0.06a | 37.9 ± 0.01e | 1770.56 ± 0.02e | 18.7 ± 0.01d | 29.03 ± 0.05d |
| p-value | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
Table 4. Mineral analyses of flour treatments, where T0, T1, T2, T3, T4 represented control, 15%, 20%, 25% and 30% of quinoa flour respectively. All values represent mean ± SE, different letters on the top suggest significant difference as per Tukey’s LSD test (P ≤ 0.05).
Ca, Mg, Na, and Fe increase significantly (P ≤ .05) because whole grain quinoa flour contains more nutrients than whole wheat flour. These essential nutrients play a crucial role in certain physiological functions. Diets enriched with these super grains play a vital role in physical wellbeing especially for malnourished community. Zn was increased from 14±0.04 to 20.11ppm ± 0.01ppm and Cu increased from 3.2 ± 0.05 to 18.7 ± 0.01 in T4. However, a decrease in mineral content was also observed. Mn and K content were reduced with the increase percentage of quinoa flour. Wheat flour itself contains more Mn and K contents when compared with Quinoa flour that’s why its decreasing percentage in composite flour blends leads to their subpar results. These micronutrients while being lesser in composite flour highlights the importance of specific percentage of all flours added which balances the overall nutritional profile so the final product must be a great source of all macro and micronutrients and if one flour lacks in any nutrient the other must fills that void.
Sensory analyses
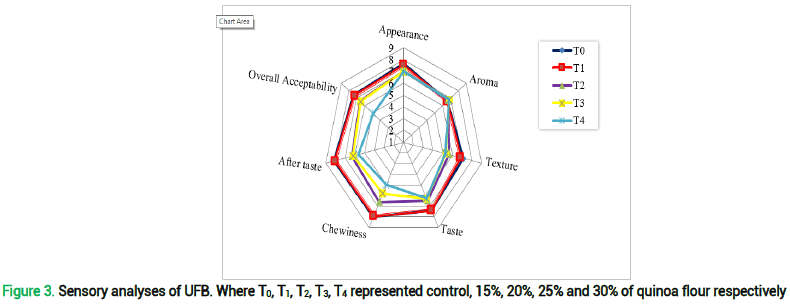
Unleavened Flat Bread (UFB) sensory assessment refers to the elements of the meal that are perceived by the senses, including taste, sight, smell, and touch. Appealing attributes of UFB are its wheatish aroma, a very vague sweet taste with good chew ability and a very soft texture. 1-9 hedonic scale (1 score; extremely dislike, 9 score; like extremely) was chosen to find out the aroma, texture, appearance, taste, after taste, chewiness and overall acceptability of UFB. Figure shows the average sensory rating of UFB baked with various quinoa-to-flour ratios. The highest overall score was given to UFBT0 which was actually the control sample while in UFB T3 it was 6.5. High insignificance was clearly observed in all UFB treatments. Overall acceptability of UFBT1-UFBT4 was reduced when compared with control UFB T0. Lowest overall acceptability was observed in UFB T4. This is clearly due to the fact that quinoa incorporation in higher percentages has inverse relation with overall acceptability as it significantly reduces it. Reason behind this is the bitterness of quinoa as well as its coarse texture which is actually unfamiliar and to some extent a little unpleasant to areas where population is fond of wheat chapatti. These sensory changes do not align with the consumer preferences which are hindrance in quinoa acceptability. But, in UFBT3, these characters are controlled to an acceptability extent.
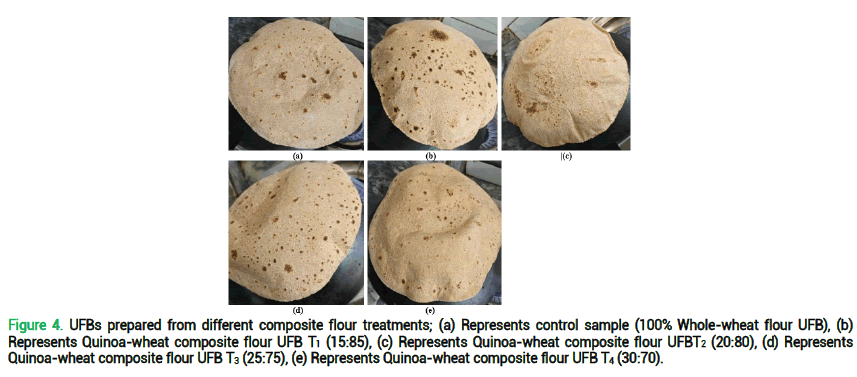
Chewiness of UFB T0 is quite close to the results of UFB T1, however it was significantly reduced in UFBT2-UFBT4 which is due to the fact that quinoa is a gluten free crop so it is deprived to develop extensible network of gluten unlike wheat and results in harder texture which leads to low chew ability. The appearance of UFB T0 scored highest which can be seen in fig. 3 and 4 (a) however the remaining four UFB (T1-T4) scored quite close to each other as figure itself explains a little dark color of UFB. Color is said to be a key factor when consumer acceptability and preferences are discussed. Consumers normally favors the lighter tone of chapatti, quinoa being a darker color grain imparts a more intense color tone which is slightly problematic when sensory analyses are performed. Leaving behind the color, overall appearance of all UFBs as seen in fig 4 a, b, c, d and e was excellent. A little difference in scores was observed in case of aroma as all the unleavened flat breads possess very vague aroma which may be due to the fact that quinoa possess earthy aroma which was masked by the aroma of wheat. The panelists gave more score to UFB T0 and UFB T1, which was obvious due to their flavor, aroma, and aftertaste. However, considering the nutritional profile and acceptable taste, UFBT3 was chosen for further in-vivo studies.
Figure 3. Sensory analyses of UFB. Where T0, T1, T2, T3, T4 represented control, 15%, 20%, 25% and 30% of quinoa flour respectively
Figure 4. UFBs prepared from different composite flour treatments; (a) Represents control sample (100% Whole-wheat flour UFB), (b) Represents Quinoa-wheat composite flour UFB T1 (15:85), (c) Represents Quinoa-wheat composite flour UFBT2 (20:80), (d) Represents Quinoa-wheat composite flour UFB T3 (25:75), (e) Represents Quinoa-wheat composite flour UFB T4 (30:70).
Although, UFBT3 does not achieved a highest score from the panellists but there were multiple reasons behind its selection for subsequent studies. First of all, even-though it shows an acceptable flavor, its nutritional profile was quite high when compared with other high scored sampled. Secondly, our target behind this research was to produce such functional UFB which not only helps to mitigate protein energy malnutrition but also ameliorate hepato and nephrotoxicity.
Results from in-vivo studies strongly support our decision to choose UFBT3 for final functional food among all the composite flour samples.
Microstructure of UFB
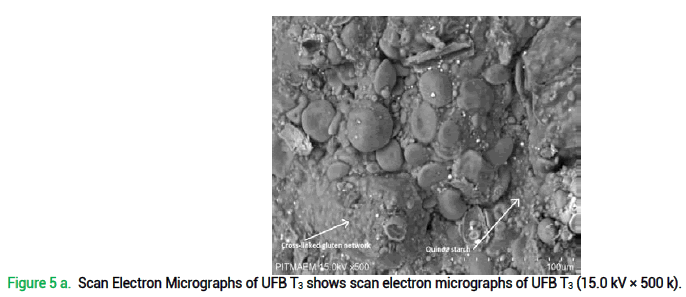
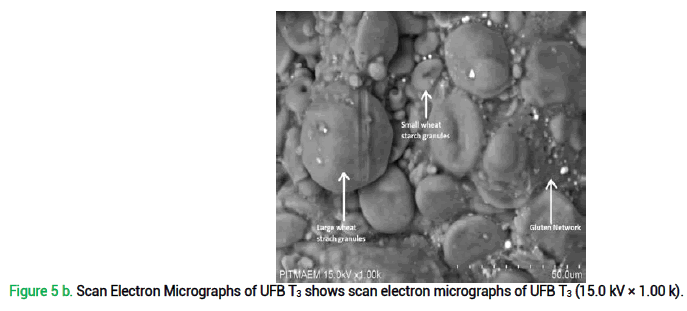
Scanning Electron Microscopy of unleavened flat bread was done to study the microstructure of gluten network. Findings of SEM may prove helpful to enhance the quality and functional application of quinoa-wheat fortified bread because quinoa flour incorporation in wheat flour clearly affects dough characteristics as its microstructure and baking performance etc. Results illustrates that quinoa flour presence in functional unleavened flat bread disrupts the gluten network as can be seen in fig. 5 where; (a) represents cross-linked gluten network developed with quinoa starch in UFB T3 detected at 500 magnifications. It clearly shows fibrous gluten network which is discontinuous and (b) shows representative SEM images of unleavened flat bread prepared with quinoa-wheat composite flour T3 at 1k magnification, showing small and large wheat starch granules. It can be stated that presence of gluten in wheat flour is responsible for gluten network development, but its discontinuity is due to the fact that quinoa incorporation, as a gluten free crop, disrupts this structure.
Figure 5 a. Scan Electron Micrographs of UFB T3 shows scan electron micrographs of UFB T3 (15.0 kV × 500 k).
Figure 5 b. Scan Electron Micrographs of UFB T3 shows scan electron micrographs of UFB T3 (15.0 kV × 1.00 k).
In-Vivo studies of functional Unleavened Flat Bread
CCl4 induced hepatotoxicity: In-Vivo studies were conducted to find out the ameliorative effect of functional unleavened flat bread T3 on rat model. Study continued for 6 weeks according to the plan mentioned in tab. 2. Rats were intoxicated by CCl4 to induce hepatotoxicity, nephrotoxicity and diabetes. Their blood was further investigated for different blood markers and histopathology of organs was done to unveil the curative effect of functional unleavened flat bread.
Liver function Test: To investigate the impact of functional unleavened flat bread on rats, serum liver biomarkers such as AST, ALP, and Total Bilirubin were measured. According to tab. 5, the hepatotoxic groups had a significant (P ≤ 0.001) increase in serum hepatic biomarkers, which indicated liver damage by CCl4. The liver biomarkers like AST and ALP reduced notably in G3 after administration of Quinoa-wheat composite chapatti (25:75) compared to G2 (CCl4 toxic group). Results clearly depict the decrease of liver enzymes in groups; G3 and G4 administered with T3 and T0. Although these values also reduced in G4 where rats were administered the control treatment (Whole wheat chapatti) but results depict a clear difference in both treatments as mentioned below. The outcomes showed a reduction in liver enzyme in the serum of the G3 and G4 groups. When compared to G2 a decrease of 64.44% was observed in G3 treatment but it was 20% in G4 treatment. Same trend was noted in ALP and total Billirubin concentration of treatment (Tab. 5). In G2 maximum (735 IU/L ± 15.01 IU/L and 1.89 mg/dL ± 0.05 mg/dL) of ALP and TB was observed respectively. Likewise, the previous trend, same observation was noted in G4, where whole wheat chapatti was given to rats. Explaining from statistical point of view, one can clearly state that significant variations were recorded among normal and toxicity induced groups. Amazing inhibitory results were observed in G3 where quinoa-wheat UFB was administered to rats which confirm the ameliorative effect of quinoa against the hepatotoxicity which confirms the functional and neutraceutical potential of Chenopodium quinoa.
| Treatment | Aspartate Aminotransferase U/L | Alkaline Phosphatase IU/L | Total Billirubin mg/dL |
|---|---|---|---|
| G1 | 17.2 ± 3.06d | 201.8 ± 0.1d | 0.74 ± 0.01d |
| G2 | 675 ± 24.5a | 735 ± 15.01a | 1.89 ± 0.05a |
| G3 | 240 ± 20.03c | 305 ± 10.1c | 1.00 ± 0.04c |
| G4 | 540 ± 30.05b | 495 ± 5.05b | 1.20 ± 0.2b |
| p-Value | 0 | 0 | 0 |
Table 5. Liver function test of rats induced with CCL4 based liver toxicity. Where G1, G2, G3 and G4 represented Normal Control, Toxic control, treated with T3 and treated with T0 respectively. All values represent mean ± SD, different letters on the top suggest significant difference as per Tukey’s LSD test (P ≤ 0.05).
Renal function tests: Regarding renal functioning biomarkers, serum urea, serum creatinine and uric acid were examined. These parameters increased logically in G2 (toxicity-induced groups) (G2; 66 mg/dL ± 4.5 mg/dL, 2.25 mg/dL ± 0.09 mg/dL, 10.11 mg/dL ± 0.21 mg/dL) when compared with G1 (normal group) (G1; 24 mg/dL ± 3.5 mg/dL, 0.81 mg/dL ± 0.05 mg/dL, 4.53 mg/dL ± 0.1 mg/dL) (Tab. 6). However, the excellent ameliorative effect was observed in G3 where, Quinoa-wheat composite chapatti (25:75) was given to rats when compared with G2 (toxicity-induced group). Therefore, G3 was more effective than the harmful control group G2 at lowering urea, creatinine and uric acid. Serum urea was reduced to 39 mg/dL ± 4.01 mg/dL in G3 and 52 mg/dL ± 4 mg/dL in G4 when compared to G2 where 66 mg/dL ± 4.5 mg/dL was recorded. Same trend was observed in serum creatinine where 2.25 mg/dL ± 0.09 mg/dL (in G2) reduced to 1.1 mg/dL ± 0.5 mg/dL in G3. G3 was more effective than the harmful control group G2 at lowering urea, creatinine and uric acid. Serum urea, creatinine and uric acid decreased up to 40.90%, 51.11% and 47.18% respectively in G3 when compared with G2 toxic group (Tab. 6). Renal biomarkers were found significantly varied (p<0.05) among different groups. CCl4 induced toxicity not only damage liver but also affects the normal activity of kidneys. These results stood firm to the fact that quinoa grains possess functional and therapeutic characteristics and its incorporation in daily life can earn multiple curative gains.
| Treatment | Urea mg/dL | Creatinine mg/Dl | Uric acid mg/dL |
|---|---|---|---|
| G1 | 24 ± 3.5d | 0.81 ± 0.05d | 4.53 ± 0.1d |
| G2 | 66 ± 4.5a | 2.25 ± 0.09a | 10.11 ± 0.21a |
| G3 | 39 ± 4.01c | 1.1 ± 0.5c | 5.34 ± 0.77c |
| G4 | 52 ± 4b | 1.9 ± 0.4b | 5.52 ± 1.2b |
| p-Value | 0 | 0.002 | 0.001 |
Table 6. Renal function test of rats induced with CCL4 based liver toxicity. Where G1, G2, G3 and G4 represented Normal Control, Toxic control, treated with T3 and treated with T0 respectively. All values represent mean ± SD, different letters on the top suggest significant difference as per Tukey’s LSD test (P ≤ 0.05).
Histopathology
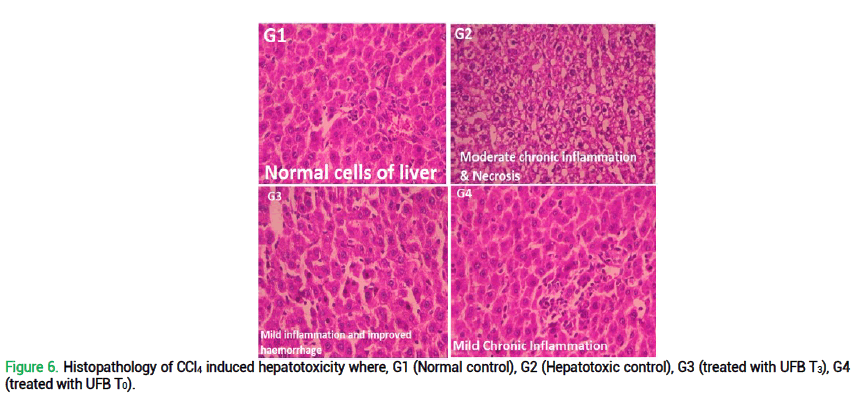
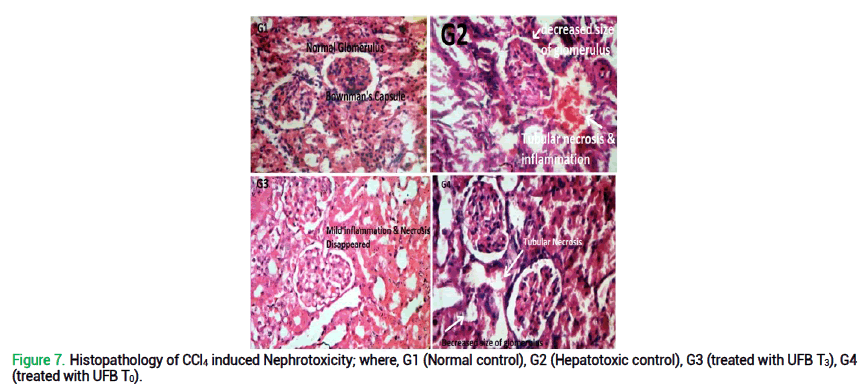
Histopathological examination of liver and kidney illustrates significant effects of quinoa-wheat UFB ingestion as show in fig. 6 and 7. Liver histopathology of G1 (normal control; no disease; provided with basal diet) presents normal cells of liver without any sign of disease, inflammation etc. However, in G2 (CCl4-induced toxic control), moderate chronic inflammation with necrosis of hepato-cytes can be clearly observed. In addition, histopathology of G3 reported mild chronic inflammation without haemorrhage and edema. While in G4, mild chronic inflammation on portal area can be observed. Renal histopathology of G1 (normal control) presents no haemorrhage or inflammation with normal renal tubes. Despite that, G2 (CCl4-induced toxic control) declares moderate inflammation with edema and tubular necrosis. Contrarily, in G3 renal tubular necrosis and haemorrhage tends to disappear. While in G4, tubular necrosis and moderate inflammation still persist. These histopathological results are consistent with the biochemical reports of rats for all liver and kidney biomarkers.
Figure 6. Histopathology of CCl4 induced hepatotoxicity where, G1 (Normal control), G2 (Hepatotoxic control), G3 (treated with UFB T3), G4 (treated with UFB T0).
Figure 7. Histopathology of CCl4 induced Nephrotoxicity; where, G1 (Normal control), G2 (Hepatotoxic control), G3 (treated with UFB T3), G4 (treated with UFB T0).
Discussion
This study scrutinize the functional potential of quinoa-wheat functional potential against CCl4 induced hepatotoxicity as golden grains like quinoa are the prime source to ameliorate multiple malfunctioning in body. Quinoa grains termed as ‘grains of 21st century’ possess great effectiveness when it comes to its health benefits claimed in literature. Nutritional profile of composite flour creates a clear difference from the control sample (whole wheat flour) and this study solidifies the fact that quinoa addition in whole wheat flour improves its nutritional profile. Our findings are consistent with those of (Villa et al. 2014) who determined the protein content of quinoa to be 11.7 ± 0.2%. Furthermore, they strongly believed that environmental influences play important roles in the presence of net protein contents. Reason behind raised protein contents is that quinoa flour itself contains an excellent protein balance when compared with whole wheat flour. Upon displacement of wheat flour with quinoa flour while making treatments, it was understood to observe high levels of protein in composite flours in comparison with control T0.
Moreover, quinoa flour had more minerals than wheat flour (Bahmanyar, Hosseini et al. 2021). In line with earlier research Demir and Kılınç (2017), the current study discovered that increasing quinoa-flour supplementation increased the amount of total fat, crude protein, and ash in composite flour. Moawad, Rizk et al. (2018) states that in comparison to wheat flour, quinoa flour had greater levels of protein, lipids, crude fiber, and ash (72% extraction), which improved the nutritional content of the combined flour and commodities (Demir and Kılınç 2017)
Mineral analyses of composite flour when compared with the control sample reveals that quinoa supplementation steadily increased the mineral contents. (Konishi et al., 2004) states that quinoa has 2200 mg kg-1 of sulphur and 40 mg kg-1 of zinc. According to González Martín et al. (2014), magnesium contents of quinoa lies between 1839.0 mg/kg ± 67.8 mg/kg and 1912.1 mg/kg ± 213.0 mg/kg. Different studies report that the application of fertilization and soil fertility may be to blame for the variance in mineral composition. However, an overall up-gradation of mineral profile was observed in current study when quinoa was added in whole wheat flour.
All the above mentioned results clearly indicates that quinoa-wheat composite flour is an outstanding substitute to whole wheat flour as its fortification leads to raised nutrients level, most precisely the protein, and the overall mineral contents which notify its consumption to protein-energy malnourished consumers.
Results of sensory evaluation clearly states that by increasing quinoa percentage in composite flour treatments, significant differences among flavor, texture and color were observed. UFB T0 scored highest and UFBT3 scored 6.5. High insignificance in all UFB treatments was observed as overall acceptability of UFB T1-UFB T4 was reduced when compared with control UFB T0. Chewiness in UFBT2-UFBT4 was significantly reduced. Park et al. (2005) substituted 30% of quinoa flour with whole wheat flour and stated the similar results which are in accordance with our findings. They conclude that absence of gluten is the leading cause behind a harder chapatti with less chew ability. Coming towards the appearance of UFBs, it was observed that quinoa incorporation in whole wheat flour leads to a darker color when compared with T0. Results aligned with the findings of El-Sohaimy et al. (2019) who observed that substituting quinoa for refined flour might modify sensory characteristics of goods, such as deeper color because of the inclusion of bran, and may influence customer choices. However, quinoa addition in UFBs leads to a slight bitter taste and can be felt as taste and aftertaste too.
This therapeutic and curative role of UFB T3 can be clearly seen in the in-vivo section in detail. Our main target of the study was to develop a functional food which helps prevent or ameliorate the hepatic and renal damage induced by CCl4. Fascinatingly, UFBT3 contains a certain amount of quinoa which is favorable to achieve the above mentioned goal. Otherwise, the chosen UFB from the panelist didn’t even contain quinoa which highlights a major gap in health concerns and consumers preferences. Hence, the demand of more palatable functional food is raised which not only satisfy the gustatory papillae of community but plays curative role as well.
Almost all previous studies conducted on quinoa incorporation in different food products like bakery, noodles, beef burgers, edible coatings, beverages etc states its nutritional gains and a very little data is available when it comes to its therapeutic or curative benefits like hepatoprotective effect against induced toxicity (Saxena et al., 2017). Our results are in accordance with the results of (Abdel-Wahhab et al., 2021) which states that administration of quinoa ethanolic extracts in cyclophosphamide induced toxicity in male rats resulted improved values and strongly endorse the hepato-protective effect of quinoa extracts against Cyclophosphamide induced toxicity as quinoa significantly lower the raised AST, ALT, GGT and ALP activities. Similarly, Saxena, Shahani et al. (2017) also studied the effect of quinoa against CCl4 induced hepatotoxicity and conclude that that quinoa seed powder significantly lessen serum level of hepatic enzymes elevated by CCl4 and restore them to their normal level. So, all these studies clearly depict the role of quinoa as a hepatoprotective agent in animals. CCl4 induced toxicity not only damage liver but also affects the normal activity of kidneys. Renal damage caused by CCl4 was studied by treating with red quinoa powder, red quinoa ethanol extract and red quinoa water extract. And the results states that red quinoa water extracts showed maximum significant results of lowering the raised Blood Urea Nitrogen (BUN) and creatinine levels (Lin et al., 2019).
In this aspect, current investigation reveals the functional and therapeutic potential of quinoa supplementation in wheat flour on renal biomarkers in lowering higher tissue enzyme levels in blood of treated animals than the animals without quinoa-wheat UFB ingestion which are in accordance with the above studies. All the previous investigations clearly conclude that improvement of liver, renal biomarkers by quinoa-wheat UFB is due to its nutritional profile and the bioactive compounds it possesses. These bioactive compounds claimed to have multiple pharmacological gains and could be account for its modulatory capacity to ameliorate induced toxicity (Graf et al., 2014).
All the above-mentioned results from current examinations strongly endorse the fact that quinoa is a super food which has the power to reverse the hepatic and renal damages induced by CCl4 and can be vouched to help prevent or cure protein energy malnutrition. These results also presents quinoa fortification in whole wheat flour an ultimate solution to malnutrition and certain chronic health conditions as hepatic and renal toxicity issues particularly in developing countries. The observations also encourage further exploration on functional foods, plant based solutions and the need to grow nutrient rich super crops.
Conclusions
This study summarizes the functional potential of quinoa incorporation in whole wheat flour for chapatti (Unleavened Flat Bread) production and analyzes its therapeutic or curative capabilities in toxicity-induced rats. The results concluded that ingestion of Quinoa-wheat functional unleavened flat bread significantly ameliorate the hepatic and renal damage just after 6 weeks. This chapatti can be useful for reducing or managing multiple clinical ailments, especially those related to protein energy malnutrition, hepatic and renal issues or toxicity. However, further investigations on “Biologically active dietary supplement” can be made by using phytoecdysteroids present in quinoa as they have the obligation to serve the pharmacological effects of quinoa.
Ethical Approval
The experimental procedures were approved by the ethical committee.
Conflict of Interest
The authors declare no conflict of interest regarding this manuscript.
References
Abbas A, Rehman AU, Javed MM. (2021). Exploring the potential of in vitro tissue culture in breeding programs of legume and pulse crops: utilization and present condition. Bull Biol Allied Sci Res. 1:36. [Goggle Scholar] [Crossref]
Abdel-Wahhab KG, Mannaa FA, Ashry M, Khaled DM, Hassan LK, Gomaa HF. (2021). Chenopodium quinoa ethanolic extract ameliorates cyclophosphamide®-induced hepatotoxicity in male rats. Comp Clin Pathol. 30:267-276. [Goggle Scholar] [Crossref]
AMIN F, Hassan N, Bashir K, KHAN A, Bibi H, Irshad M, Khan S, Nawaz K, Ullah Z. (2023). Antimicrobial susceptibility profile of various bacteria isolated from respiratory tract infection. Allied Sci Res. 1:48. [Goggle Scholar] [Crossref]
Azzane A, Amssayef A, Eddouks M. (2022). Chenopodium quinoa exhibits antihyperglycemic activity in streptozotocin-induced diabetic rats. Cardiovasc Hematol Agents Med Chem (Former Curr MCedicinal Chem-Cardiovasc Hematol Agents. 20:125-132. [Goggle Scholar] [Crossref]
Bancroft JD, Gamble M. (2008). Theory and practice of histological techniques. Elsevier health Sci. [Goggle Scholar] [Crossref]
Baur FJ, Ensminger LG. (1977). The association of official analytical chemists (AOAC). J Am Oil Chem Soc. 154:171-172. [Goggle Scholar] [Crossref]
Din SZ, Fazal MO, Ishtiaque A, Ullah A. (2023). Antimicrobial activity of lantana camara against pseudomonas aeruginosa, serratia marcescens and staphylococcus aureus to develop ointment based therapy. Bull Biol Allied Sci Res. 33. [Goggle Scholar] [Crossref]
El-Sohaimy SA, Shehata MG, Mehany T, Zeitoun MA. (2019). Nutritional, physicochemical, and sensorial evaluation of flat bread supplemented with quinoa flour. Int J Food Sci. 2019:4686727. [Goggle Scholar] [Crossref]
Fawcett J, Scott J (1960). A rapid and precise method for the determination of urea. J Clin Pathol. 13:156-159. [Goggle Scholar] [Crossref]
Fuentes F, Paredes-Gónzalez XI. (2013). Nutraceutical perspectives of quinoa: biological properties and functional applications. FAO CIRAD: state art rep quinoa world in. 2013:286-299. [Goggle Scholar]
González Martín MI, Wells Moncada G, Fischer S, Escuredo O. (2014). Chemical characteristics and mineral composition of quinoa by nearâ?infrared spectroscopy. J. Sci. Food Agric. 94:876-881. [Goggle Scholar] [Crossref]
Graf BL, Poulev A, Kuhn P, Grace MH, Lila MA, Raskin I. (2014). Quinoa seeds leach phytoecdysteroids and other compounds with anti-diabetic properties. Food Chem. 163:178-185. [Goggle Scholar] [Crossref]
Haider MZ, Sami A, Mazhar HS, Akram J, Nisa BU, Umar M, Meeran MW. (2023). Exploring morphological traits variation in Gomphrena globosa: A multivariate analysis. Biol Agric Sci Res J. 21. [Goggle Scholar] [Crossref]
Ihekoronye AI, Ngoddy PO (1985). Integrated food science and technology for the tropics. [Goggle Scholar]
Javed MM, Sami A, Haider M, Abbas A, Ali M, Naeem S, Amjad M, Ahmad A, Bostani R. (2024). The contribution of transgenic rice to enhance grain yield. Bull Biol Allied Sci Res. 65. [Goggle Scholar] [Crossref]
Junaid MD, Gokce AF (2024). Global agricultural losses and their causes (2024). Bull Biol Allied Sci Res. 66. [Goggle Scholar]
Konishi Y, Hirano S, Tsuboi H, Wada M. (2004). Distribution of minerals in quinoa (Chenopodium quinoa Willd.) seeds. Biosci biotechnol biochem. 68:231-234. [Goggle Scholar] [Crossref]
Lin TA, Ke BJ, Cheng CS, Wang JJ, Wei BL, Lee CL. (2019). Red quinoa bran extracts protects against carbon tetrachloride-induced liver injury and fibrosis in mice via activation of antioxidative enzyme systems and blocking TGF-β1 pathway. Nutrients. 11:395. [Goggle Scholar] [Crossref]
Moraes ÉA, Natal DI, Queiroz VA, Schaffert RE, Cecon PR, de Paula SO, dos Anjos Benjamim L, Ribeiro SM, Martino HS. (2019). Sorghum genotype may reduce low-grade inflammatory response and oxidative stress and maintains jejunum morphology of rats fed a hyperlipidic diet. Food Res Int. 49:553-559. [Goggle Scholar] [Crossref]
Park SH, Maeda T, Morita N. (2005). Effect of whole quinoa flours and lipase on the chemical, rheological and breadmaking characteristics of wheat flour. J Appl Glycosci. 52:337-343. [Google Scholar][Crossref]
Pasko P, Barton H, Zagrodzki P, Izewska A, Krosniak M, Gawlik M, Gawlik M, Gorinstein S. (2010). Effect of diet supplemented with quinoa seeds on oxidative status in plasma and selected tissues of high fructose-fed rats. Plant Foods Hum Nutr. 65:146-151. [Google Scholar][Crossref]
Prabhasankar P, Indrani D, Rajiv J, Rao GV. (2003). Scanning electron microscopic and electrophoretic studies of the baking process of south Indian parotta—an unleavened flat bread. Food Chem. 82:603-609. [Google Scholar][Crossref]
Rasool GH, Anjum FM, Butt MS (2005). Bioevaluation of oilseed enriched wheat chapaties. Int J Agri Biol. 7:663-667. [Goggle Scholar]
Saxena SN, Shahani LA, Bhatnagar PR (2017). Hepatoprotective effect of Chenopodium quinoa seed against CCl4-induced liver toxicity in Swiss albino male mice. Asian J Pharm Clin Res. 10:273-276. [Goggle Scholar]
Stikic R, Glamoclija D, Demin M, Vucelic-Radovic B, Jovanovic Z, Milojkovic-Opsenica D, Jacobsen SE, Milovanovic M (2012). Agronomical and nutritional evaluation of quinoa seeds (Chenopodium quinoa Willd.) as an ingredient in bread formulations. J Cereal Sci. 55:132-138. [Goggle Scholar] [Crossref]
Tacer-Caba Z, Nilufer-Erdil D, Ai Y (2015). Chemical composition of cereals and their products. Handb Food Chem. 1:301-329. [Goggle Scholar]
Ullah W, Ullah A, Khan MA, Hassan N, Aman K, Khan S, Hassan S, Hazrat A (2023). Microbial profile and nutritional evaluation of broiler and domestic chicken meat from selected districts of Khyber Pakhtunkhwa, Pakistan. Bull Biol Allied Sci Res. 34. [Goggle Scholar] [Crossref]
Vilche C, Gely M, Santalla E (2003). Physical properties of quinoa seeds. Biosyst Eng. 86:59-65. [Goggle Scholar] [Crossref]
Villa DY, Russo L, Kerbab K, Landi M, Rastrelli L (2014). Chemical and nutritional characterization of Chenopodium pallidicaule (cañihua) and Chenopodium quinoa (quinoa) seeds. Emir J Food Agric. 26:609. [Goggle Scholar]
Yaseen AA, Shouk AE, Selim MM. (2007). Egyptian balady bread and biscuit quality of wheat and triticale flour blends. Polish journal of food and nutrition sciences. 57:25-29. [Google Scholar]