Research Article - Modern Phytomorphology ( 2025) Volume 19, Issue 5
Essential oil composition, antibacterial activity and postharvest application of Portulacaria afra essential oil for enhancing shelf life and quality of citrus fruits
Moazzam Anees1, Muhammad Rizwan Tariq2, Muhammad Saleem Haider3 and Azeem Intisar4*2Department of Food Sciences, Faculty of Agricultural Sciences, University of the Punjab, Quaid-e-Azam Campus, Lahore, 54590, Pakistan
3Department of Plant Pathology, Faculty of Agricultural Sciences, University of the Punjab, Quaid-e-Azam Campus, Lahore, 54590, Pakistan
4School of Chemistry, University of the Punjab, Lahore, 54590, Pakistan
Azeem Intisar, School of Chemistry, University of the Punjab, Lahore, 54590, Pakistan, Email: azeemintisar.chem@pu.edu.pk
Received: 09-Sep-2025, Manuscript No. mp-25-170912; Accepted: 06-Nov-2025, Pre QC No. mp-25-170912 (PQ); Editor assigned: 11-Sep-2025, Pre QC No. mp-25-170912 (PQ); Reviewed: 23-Oct-2025, QC No. mp-25-170912; Revised: 04-Nov-2025, Manuscript No. mp-25-170912 (R); Published: 13-Nov-2025, DOI: 10.5281/zenodo.17605573
Abstract
The current study examines the extraction of the essential oil from Portulacaria afra, with a particular focus on its use as a coating material to enhance the postharvest attributes and shelf life of citrus (citrus reticulata). Essential Oils (EOs) were obtained through the microwave assisted distillation process and analyzed by means of Gas Chromatography-Mass Spectrometry (GC-MS), which demonstrated a wide variety of bioactive compounds. Notably, twenty-nine compounds were successfully identified in the extracted essential oil. The main constituents were palmitic acid (36.95%), linoleic acid (8.83%), and 5-methyl-2-furancarboxaldehyde (8.82%). Antibacterial assays demonstrated significant inhibitory effects of P. afra oil against Escherichia coli and Staphylococcus aureus, supporting its potential application as natural antimicrobial agent. Subsequently, the essential oils were formulated into coatings and applied to citrus fruits. The coated fruits were assessed for a comprehensive array of postharvest quality parameters, including visual appearance, color, aroma, gloss, taste, firmness, weight loss, titratable acidity, pH, ascorbic acid content, total soluble solids, and the activities of antioxidant enzymes such as Superoxide Dismutase (SOD), Peroxidase (POD), and Catalase (CAT). The findings demonstrated that all the essential oil coatings were very effective in delaying senescence, maintenance of organoleptic properties, and microbial spoilage relative to the untreated controls. P. afra coating showed better behavior in most parameters, especially in terms of maintaining firmness, lessening weight loss, increasing vitamin C retention and controlling oxidative stress by increasing POD and CAT activity.
Keywords
Portulacaria afra, Citrus, essential oil, Postharvest, Shelf life, Antimicrobial
Introduction
Portulacaria afra belongs to the Didieraceae, traditionally known as the Portulacaceae, a family of plants, either succulent or woody, that are native to South Africa, and usually grow in semi-arid areas (Du Toit, et al. 2023). It possesses different classes of com-pounds such as terpenoids, steroids, phenols, coumarins, flavonoids, saponins and alkaloids (Mhosva, et al. 2024). Pharmacologically, these compounds are known to exhibit antimicrobial, antioxidant, antiinflammatory, antinociceptive, and tyrosinase inhibitory activities (Tabassum, et al. 2023, Basson, et al. 2023). Previously, the plant was considered primarily ornamental, but its medicinal properties and nutritional value have now been recognized (Tabassum, et al. 2022).
Citrus fruits (e.g., lemons, oranges, and mandarins) are not only culinary delicacies but also commercially significant crops cultivated as valuable agricultural investments. To achieve healthy and high yields, growers must optimize all aspects of cultivation, including water management, seed quality, and adoption of efficient agronomic practices (Hewett, 2006, Kameswara Rao, et al. 2017). Applying essential oil-based coatings to citrus fruits represents a novel postharvest strategy for prolonging shelf life and maintaining fruit quality. Due to their inherent antifungal and antibacterial properties, these oils not only extend marketability but also preserve the structural and sensory integrity of the fruits. When combined with other naturally occurring bio-compounds, they provide an environmentally friendly alternative to synthetic fungicides (Ikarini, et al. 2025).
Essential Oils (EOs) have been extensively studied for their potent antibacterial and antifungal activities (Bolouri, et al. 2022). Therefore, there is growing scientific interest in essential oils for their applications in both agricultural and health sectors. In addition to these beneficial effects, essential oils can also be used as edible coatings for food preservation (Sánchez-González, et al. 2011, Antonino, et al. 2024). The major objective of this research was to produce the stem essential oil of the plant in the first time and report on its chemical composition and antibacterial activity. Another method that was included in the study was the extraction by a modern method of extraction (microwave assisted extraction) that was followed by separation and characterization. Moreover, the EO coatings were prepared and utilized in the study of their efficiency in maintaining quality and freshness of citrus fruit.
Materials and Methods
Plant material collection and oil extraction
Fresh samples of Portulacaria afra were collected from Lahore, Pakistan, and a voucher specimen was authenticated and deposited. Essential oils were extracted from 100 g of plant material using microwave assisted distillation regulated at 60% power with a modified domestic microwave (Orient OM46SS, 230 V, 1000 W). Dichloromethane was used as the collecting solvent, and the obtained oils were dried, transferred to pre-weighed vials, and stored at -10°C for further analyses.
Gas Chromatography-Mass Spectrometry (GC-MS)
Essential oil composition was analyzed using GC-MS (Shimadzu 2030, QP2020 NX detector). A DB-5MS column (30 m × 0.25 mm × 0.25 μm) was used, with helium as the carrier gas (1 mL/min). The column temperature was programmed from 50°C (4 min hold) to 280°C at 7°C/min. Injection volume was 1 μL (split ratio 1:1). Spectra were recorded in the m/z range 35-500, with transfer line, ion source, and quadrupole at 280°C, 230°C, and 150°C, respectively. Retention Indices were calculated using standard alkanes (C7-C30) and identified by NIST library matching. Compounds were listed in the increasing order of retention time and index.
Antibacterial assay
The antibacterial activity of P. afra oil was tested by the disc diffusion method. Strains included E. coli, P. aeruginosa, S. aureus, and Bacillus spp., obtained from IMMG, University of the Punjab. Petri plates with Luria-Bertani agar were inoculated with bacterial suspensions. Sterile discs were loaded with 25 μL of essential oil (250 mg in 200 μL DMSO). Cefadroxil monohydrate served as a positive control. Plates were incubated at 37°C for 24 h, and zones of inhibition were measured in mm.
Development and application of coatings
Freshly harvested citrus reticulata cv. (Kinnow) fruits were selected at horticultural maturity and transported hygienically to the Department of Horticulture, University of the Punjab. Upon arrival, the fruits were thoroughly cleaned with distilled water, shade-dried, and sorted for uniformity in terms of size, shape, and external integrity. Only visually healthy and blemish-free fruits were included in the study. Formulation of coatings were made using EOs which were dissolved in carrier solvents and mixed thoroughly to obtain uniformity and homogeneous dispersion. They were treated by the dipping technique, and the fruits were immersed in one minute of treatment of a fixed time. The small batches (3-4 fruits in a set) were used to treat fruits so that they received a uniform coating. The fruits were then laid on sanitized mesh trays to dry at room temperature in order to create a solid layer on the fruit surface interfering with inconsistency in application. These were then kept in a special storage room at 25 to 30°C, which is the typical room temperature.
Data collection and analytical approach
Analyses were performed at 0, 2, 4, 6, 8, and 10 days of storage. Fruits appearance, aroma, gloss, taste and acceptability (9-point scale) were parameters. Weight loss, firmness (Lu and Peng, 2006), titratable acidity (citric acid equivalent), pH (pH meter), ascorbic acid content, total soluble solids and enzymatic activities (SOD, POD, and CAT) (Hadwan and Ali, 2018). Data were analyzed by one-way ANOVA to assess the effects of coating and storage duration. Post hoc tests were applied where needed. Significance was set at p<0.05.
Results and Discussion
Chemical composition of Portulacaria afra essential oil
The stem oil yield of Portulacaria afra was 0.025% (w/w). The GC-MS analysis was able to identify 29 compounds which is a wide range of bioactive classes such as furanoids, aromatic aldehydes, ketones, phenolics, fatty acids, esters, and terpenoids etc. They included palmitic acid (36.95%), linoleic acid (8.83%), and 2-furancarboxaldehyde, 5-methyl (8.82%). Numerous other bioactive constituents like carvacrol, estragole, anethole and methyl salicylate were also identified in low quantities (0.10-2.26%). The entire list of identified constituents, arranged in the order of increasing retention times is provided in Table 1 along with their retention Indices (calculated: RIcal and literature: RIlit).
| S. No | Retention time | Constituents of essential oil | Calculated Retention Indices (RIcal) | Literature Retention Indices (RIlit) |
|---|---|---|---|---|
| 1 | 6.35 | Ethanone, 1-(2-furanyl)- | 921 | 925 |
| 2 | 7.69 | 2-Furancarboxaldehyde, 5-methyl- | 976 | 970 |
| 3 | 9.35 | 2,5-Furandione, 3,4-dimethyl- | 1042 | 1038 |
| 4 | 9.65 | Benzeneacetaldehyde | 1054 | 1053 |
| 5 | 10.48 | Methyl pyrrol-2-yl ketone | 1087 | 1075 |
| 6 | 12.02 | Benzyl nitrile | 1149 | 1150 |
| 7 | 12.24 | Camphor | 1158 | 1156 |
| 8 | 13.36 | Methyl salicylate | 1204 | 1203 |
| 9 | 13.47 | Estragole | 1209 | 1206 |
| 10 | 15.32 | Nonanoic acid | 1287 | 1283 |
| 11 | 15.57 | Anethole | 1298 | 1288 |
| 12 | 15.73 | Carvacrol | 1305 | 1299 |
| 13 | 16.14 | 2-Methoxy-4-vinylphenol | 1323 | 1324 |
| 14 | 17.06 | 3-Allylguaiacol | 1365 | 1362 |
| 15 | 17.47 | n-Decanoic acid | 1383 | 1387 |
| 16 | 18.06 | Vanillin | 1410 | 1410 |
| 17 | 19.71 | 1,2,4-Cyclopentanetrione, 3-butyl- | 1489 | 1486 |
| 18 | 20.83 | Dihydroactinidiolide | 1545 | 1548 |
| 19 | 21.38 | Nerolidol | 1569 | 1565 |
| 20 | 21.57 | Lauric acid | 1583 | 1580 |
| 21 | 25.25 | Myristic acid | 1781 | 1777 |
| 22 | 25.33 | Benzyl Benzoate | 1785 | 1785 |
| 23 | 26.52 | Hexahydrofarnesyl acetone | 1854 | 1849 |
| 24 | 26.95 | Pentadecanoic acid | 1879 | 1873 |
| 25 | 27.92 | Palmitic acid, methyl ester | 1938 | 1938 |
| 26 | 28.28 | Isophytol | 1960 | 1950 |
| 27 | 29.21 | Palmitic acid | 2018 | 2010 |
| 28 | 31.72 | Linoleic acid | 2182 | 2179 |
| 29 | 32.00 | Stearic acid | 2201 | 2200 |
Table 1. Chemical components of the essential oil of stem of P. afra.
Antibacterial activity of essential oils
The antibacterial activity of the essential oil was evaluated using the agar disc diffusion assay. P. afra stem oil displayed moderate antibacterial activity (Table 2) against Staphylococcus aureus (11 mm) and Escherichia coli (12 mm). No inhibitory effect was observed against Bacillus sp. The positive control (cefadroxil monohydrate) demonstrated a strong inhibitory zone (38 mm) against Bacillus sp., but showed lower or no activity against other strains, highlighting the comparative potential of plant-derived essential oils. The antimicrobial effect is likely due to the presence of several well-known antibacterial compounds, including palmitic acid, linoleic acid, stearic acid, carvacrol, estragole, and anethole (Auezova, et al. 2020, Choi, et al. 2013, Huguet, et al. 2022, Khwaza and Aderibigbe, 2025, Song, et al. 2016, Yff, et al. 2002). The antimicrobial action may also be attributed to the synergistic effect of multiple compounds, which damage bacterial membranes and inhibit key metabolic enzymes (MÄ czka, et al. 2023).
| Sample Name | Escherichia coli | Pseudomonas aeruginosa | Staphylococcus aureus | Bacillus |
|---|---|---|---|---|
| P. afra | 12 | 10 | 11 | - |
| Negative control (DMSO) 25μL |
11 | 10 | 10 | - |
| Positive control (Cefadroxil Monohydrate) 20μL |
12 | - | - | 38 |
Table 2. Antibacterial activity of Portulacaria afra stem essential oil. Zone of inhibition (mm) measured using 25 μL of test solution (concentration= 250 mg oil / 0.2 mL DMSO).
Impact of coatings on sensory parameters of citrus (Kinnow) fruits at room temperature under different storage days
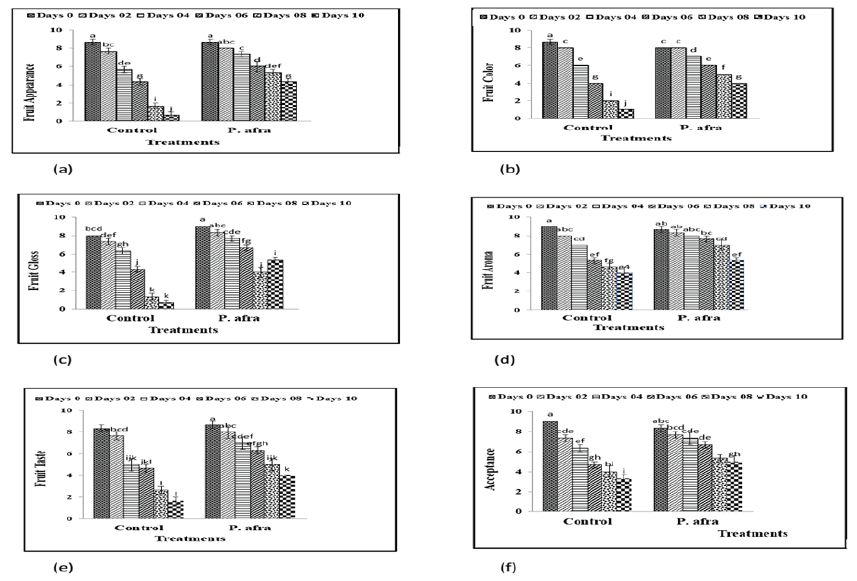
Analysis of the visual appearance of citrus fruits revealed a highly significant difference among treatments at room temperature, as illustrated in Figure 1a. Coating treatments and storage duration significantly influenced (p ≤ 0.05) the visual appearance of citrus fruits. Fruit visual appearance consistently decreased from Day 0 to Day 10 during storage at room temperature. Coating and storage time significantly (p ≤ 0.05) affected the color of citrus fruits. Fruit color showed a gradual and significant change from Day 0 to Day 10 in the control treatment during storage at room temperature (Figure 1b). Statistical analysis of fruit color revealed a highly significant difference among the treatments. The control fruits lost their edible quality after 10 days of storage at room temperature. Analysis of fruit gloss data revealed a highly significant difference among treatments. The fruit gloss of control and coated fruits under different storage durations at room temperature is presented in Figure 1c. Statistical analysis of fruit aroma revealed a highly significant difference among the treatments (Figure 1d). Coating treatments and storage time significantly (p ≤ 0.05) affected the aroma of citrus fruits. Fruit aroma gradually decreased from Day 0 to Day 10 during storage at room temperature (Figure 1d). Coating treatment and storage time significantly (p ≤ 0.05) influenced the taste of citrus fruits. Fruit taste deteriorated progressively from Day 0 to Day 10 during storage at room temperature (Figure 1e). Coating treatments and storage time significantly (p ≤ 0.05) affected fruit acceptability (Figure 1f). Fruit acceptability gradually decreased from Day 0 to Day 10 during storage at room temperature.
Figure 1: Effect of different coatings on fruit appearance (a), color (b), gloss (c), aroma (d), taste (e) and acceptance (f) in citrus (kinnow) fruits at room temperature under different storage days.
Impact of coating on physicochemical attributes of citrus (kinnow) fruits at room temperature under different storage days
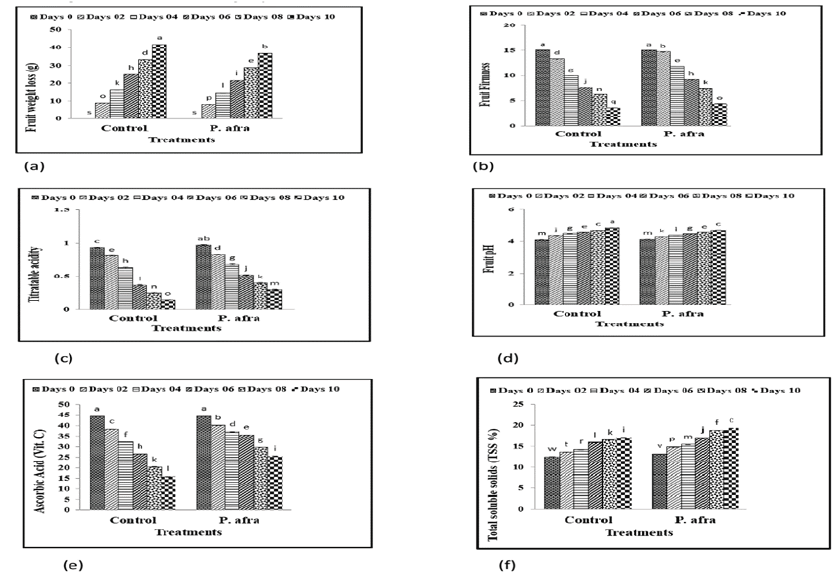
Statistical analysis of the data on fruit weight loss revealed a highly significant difference as compared to control. Coating treatment and storage time significantly (P ≤ 0.05) improved the fruit weight loss of citrus fruits (Figure 2a). The coating with P. afra remained firmer than the citrus fruit coated with any other coatings. The lowest fruit firmness was recorded in the control group, which did not receive any treatment. As illustrated in Figure 2b, the application of coatings significantly enhanced fruit firmness compared to the untreated control. The data regarding titratable acidity revealed that there is a highly significant difference among treatments as shown in Figure 2c. Titratable acidity of citrus fruits was significantly (p<0.05) affected by different coatings and the storage period. Collected data on the pH of citrus fruits at room temperature under different storage days was subjected to statistical analysis to find out any variation among the treatments. Citrus fruits treated with P. afra, had significantly improved the fruit pH on different days at room temperature storage (Figure 2d). Fruit ascorbic acid of citrus fruits under room temperature storage was significantly (p<0.05) affected by coating and storage period. The control citrus fruits showed significant (p<0.05) decline in fruit ascorbic acid level that decrease maximum on day 10; though, coating treatments significantly (p<0.05) increase in vitamin C level of citrus fruits during the storage period from Day 0 to Day 10 (Figure 2e). TSS of citrus fruits under room temperature storage was significantly (p<0.05) affected by both parameters. On the other hand, the citrus fruit control demonstrated a significant (p<0.05) increase in TSS that reached the peak value on day 10; though, coating treatments considerably (p<0.05) increase the TSS of citrus fruits during the storage period from Day 0 to Day 10 (Figure 2f).
Figure 2: Effect of coating on fruit weight loss (a), firmness (b), titratable acidity (c), pH (d), ascorbic acid (e) and total soluble solids (f) in citrus (Kinnow) fruits at room temperature under different storage days.
Antioxidant enzyme assays
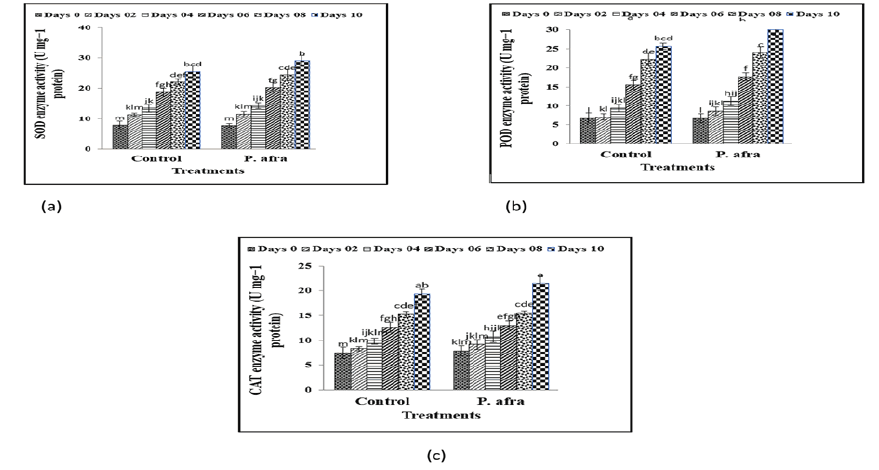
Coating treatment remarkably (p ≤ 0.05) influenced the SOD activity of citrus fruit at room temperature under 10 days storage period (Figure 3a). SOD activity exhibited a rising trend at room temperature up to the 10th day of storage in the control treatment. A decrease in SOD activity was initiated in the citrus fruits treated with different concentrations of P. afra (Figure 3a). SOD showed a remarkable (p ≤ 0.05) decline in its activity in coated treatment at room temperature as compared to control treatment. Coating treatment remarkably (p ≤ 0.05) affected the POD activity of citrus fruit at room temperature under 25 days storage period. POD exhibited a significant (p ≤ 0.05) rise in its activity in all treatments during the whole storage period at room temperature (Figure 3b). As for the activity of CAT enzyme, there was significant difference among coating treatments and control up to the 25 days of the storage period (Figure 3c). CAT exhibited a significant (p ≤ 0.05) rise in its activity in P. afra treatment during the whole storage period at room tem perature. The maximum CAT enzyme activity was showed by P. afra, as compared to control after 10 days of storage period. The volatile components were found to play a crucial role in enhancing the overall aroma stability of the coated fruits. The cold storage environment led to a reduction in fruit firmness, likely due to pectin and cell wall depolymerization, increased ethylene synthesis, and moisture loss. These findings are consistent with Tahir, et al. 2018, who reported that edible coatings extend the shelf life of strawberries by inhibiting cell wall-degrading enzymes and reducing water vaporization. Vitamin C (ascorbic acid), known for its sensitivity to heat and oxygen, showed a significant decline in untreated samples. However, essential oil encapsulation effectively mitigated this loss. Fruits treated with P. afra retained the highest ascorbic acid levels, confirming the antioxidant protection provided by its phytochemical constituents (Mditshwa, et al. 2017). Total Soluble Solids (TSS) increased with storage duration across all treatments, reflecting normal ripening associated sugar accumulation. However, a greater TSS increase in P. afra-treated fruits suggests reduced respiration and metabolic activity, which preserves sugars. Gu, et al. 2024 have observed that TSS increases throughout the storage process, and these are attributed to the breakdown of starch, turning it into glucose, fructose, and sucrose and to the loss of water. In terms of enzyme activity, SOD activity of control fruits increased with oxidative stress whereas coated fruits had low SOD activity which indicated less stress. Conversely, the coated samples depicted an increment in the POD activity that suggests increment in the hydrogen peroxide scavenging. P. afra coatings were identified to be particularly interactive in regards to POD and CAT in which CAT breaks down the hydrogen peroxide to oxygen and water, eliminating the oxidative stress. Improved tissue integrity and antioxidant defense and delayed senescence were associated with the synergistic activation of POD, SOD, and CAT (Wang, et al. 2019). In general, the P. afra essential oils increased the antioxidant activity of fruits, including the peroxidase, superoxide dismutase, and catalase enzyme activity, which increased the shelf life and preserved the postharvest quality.
Figure 3: Effect of coating on Superoxide Dismutase (SOD) activity (a), peroxidase (POD) activity (b) and catalase (CAT) activity (c) in citrus (Kinnow) fruits at room temperature under different storage days.
Conclusion
The study contains sufficient data regarding the microwave extraction, chemical profiling, and antimicrobial potential of Portulacaria afra essential oils. A total of 29 constituents were identified by GC-MS analysis including those exhibiting antioxidant and antimicrobial properties. Postharvest quality indicators of essential oil coatings demonstrated significantly better values than the uncoated control fruits. The coated fruits revealed the improved biochemical properties including vitamin C (ascorbic acid), titratable acidity and pH. The SOD, POD and CAT were also affected positively by the coatings. Therefore, these essential oils revealed a significant potential as a natural coating material for extending the shelf life and quality of fruit during storage.
Acknowledgement
All the assistance is accorded by the University of the Punjab, Lahore.
Conflict of Interest
Authors state that they do not have any conflict of interest among one another.
References
- Antonino C, Difonzo G, Faccia M, Caponio F. (2024). Effect of edible coatings and films enriched with plant extracts and essential oils on the preservation of animalâderived foods. J Food Sci. 89:748-772.
[Crossref] [Google Scholar] [PubMed]
- Auezova L, Najjar A, Kfoury M, Fourmentin S, GreigeâGerges H. (2020). Antibacterial activity of free or encapsulated selected phenylpropanoids against Escherichia coli and Staphylococcus epidermidis. J Appl Microbiol. 128:710-720.
[Crossref] [Google Scholar] [PubMed]
- Basson DC, Teffo TK, Risenga IM. (2023). A phytochemical screening, antioxidant and antibacterial activity analysis in the leaves, stems and roots of Portulacaria afra. J Herbmed Pharmacol. 12:109-117.
- Bolouri P, Salami R, Kouhi S, Kordi M, Lajayer BA, Hadian J, Astatkie T. (2022). Applications of essential oils and plant extracts in different industries. Molecules. 27:8999.
[Crossref] [Google Scholar] [PubMed]
- Choi J-S, Park N-H, Hwang S-Y, Sohn JH, Kwak I, Cho KK, Choi IS. (2013). The antibacterial activity of various saturated and unsaturated fatty acids against several oral pathogens. J Environ Biol. 34:673.
[Google Scholar] [PubMed]
- Du Toit A, MacDonald R, Steyn E, Mahlanza ZP, Zulu AB, De Wit M. (2023). Review of the underutilized indigenous Portulacaria afra (spekboom) as a sustainable edible food source. Agronomy. 13:1206.
- Gu S, Jing M, Li D, Ma Z, Duan Y, Wang L, Dai X, Chen Z, Zhang X, Chen J. (2024). The effects of different temperature and humidity conditions on the ripening and cracking of Annona atemoya fruit during storage by regulating the conversion of starch into soluble sugars. LWT. 208:116703.
- Hadwan MH, Ali SK. (2018). New spectrophotometric assay for assessments of catalase activity in biological samples. Anal Biochem. 542:29-33.
[Crossref] [Google Scholar] [PubMed]
- Hewett EW. (2006). An overview of preharvest factors influencing postharvest quality of horticultural products. Int J Postharvest Technol. 1:4-15.
- Huguet, C, Bourjot M, Bellanger J-M, Prévost G, Urbain, A. (2022). Screening for antibacterial activity of French mushrooms against pathogenic and multidrug resistant bacteria. Appl Sci. 12:5229.
- Ikarini I, Hamaisa A, Fitriani F, Nur M, Al Hakim FP, Kurniawati IMF, Lesmayati S, Triasih U, Triwiratno A, Dwiastuti ME. (2025). Chitosan based edible coating reinforced with citrus mandarin cv. terigas essential oil for shelf life extension and green mold prevention in Nagami Kumquats. RINENG. 105993.
- Kameswara Rao N, Dulloo ME, Engels JM. (2017). A review of factors that influence the production of quality seed for long-term conservation in genebanks. Genet Resour Crop Evol. 64:1061-1074.
- Khwaza V, Aderibigbe BA. (2025). Antibacterial activity of selected essential oil components and their derivatives: A review. Antibiotics. 14:68.
[Crossref] [Google Scholar] [PubMed]
- Lu R, Peng Y. (2006). Hyperspectral scattering for assessing peach fruit firmness. Biosystems Engineering. 93:161-171.
- MÄ
czka W, Twardawska M, Grabarczyk M, WiÅska K. (2023). Carvacrol-A natural phenolic compound with antimicrobial properties. Antibiotics. 12:824.
[Crossref] [Google Scholar] [PubMed]
- Mhosva Y, Nkomozepi P, Nalla S, Nyakudya T. (2024). Pharmacological potential of Portulacaria afra: A review on bioactive compounds, pharmacological uses and therapeutic prospects. Sci Afr. 26:e02491.
- Mditshwa A, Magwaza LS, Tesfay SZ, Opara UL. (2017). Postharvest factors affecting vitamin C content of citrus fruits: A review. Sci Hortic. 218:95-104.
- Sánchez-González L, Vargas M, González-Martínez C, Chiralt A, Chafer M. (2011). Use of essential oils in bioactive edible coatings: A review. Food Eng Rev. 3:1-16.
- Song YR, Choi MS, Choi GW, Park IK, Oh CS. (2016). Antibacterial activity of cinnamaldehyde and estragole extracted from plant essential oils against Pseudomonas syringae pv. actinidiae causing bacterial canker disease in kiwifruit. Plant Pathol J. 32:363.
[Crossref] [Google Scholar] [PubMed]
- Tabassum S, Ahmad S, Khan KR, Ali B, Usman F, Jabeen Q, Sajid-ur-Rehman M, Ahmed M, Zubair HM, Alkazmi L, Batiha GE. Chemical profiling and evaluation of toxicological, antioxidant, anti-inflammatory, anti-nociceptive and tyrosinase inhibitory potential of Portulacaria afra using in-vitro, in-vivo and in-silico studies. Arab J Chem. 16:104784.
[Crossref]
- Tabassum S, Ahmad S, Rehman KY, Khurshid U, Rao H, Alamri A, Ansari M, Ali B, Waqas M, Saleem H. (2022). Phytochemical, biological, and in-silico characterization of Portulacaria afra Jacq.: A possible source of natural products for functional food and medicine. S Afr J Bot. 150:139-145.
- Tahir HE, Xiaobo Z, Jiyong S, Mahunu GK, Zhai X, Mariod AA. (2018). Quality and postharvestâshelf life of coldâstored strawberry fruit as affected by gum arabic (Acacia senegal) edible coating. J Food Biochem. 42:e12527.
- Wang L, Ning T, Chen X. (2019). Postharvest storage quality of citrus fruit treated with a liquid ferment of Chinese herbs and probiotics. Sci Hortic. 255:169-174.
- Yff BT, Lindsey KL, Taylor MB, Erasmus DG, Jäger AK. (2002). The pharmacological screening of Pentanisia prunelloides and the isolation of the antibacterial compound palmitic acid. J Ethnopharmacol. 79:101-107.
[Crossref] [Google Scholar] [PubMed]