Research - Modern Phytomorphology ( 2025) Volume 19, Issue 1
Characterization and bioactivity of carrot (Daucus carota L. subsp. sativus (Hoffm.) Arcang by Supercritical Liquid CO2 Extraction
Zeliha Ustun Argon1Department of Biotechnology, Faculty of Science, Necmettin Erbakan University, Konya, Turkey
2Medical and Cosmetic Plants Application and Research Center, Konya, Turkey
3Department of Biology, Faculty of Science, Selcuk University, Konya, Turkey
Suleyman Dogu, Medical and Cosmetic Plants Application and Research Center, Konya, Turkey, Email: sdogu@erbakan.edu.tr
Received: 05-Sep-2024, Manuscript No. mp-25-147465; Accepted: 06-Oct-2024, Pre QC No. mp-25-147465 (PQ); Editor assigned: 08-Sep-2024, Pre QC No. mp-25-147465 (PQ); Reviewed: 24-Sep-2024, QC No. mp-25-147465 (Q); Revised: 30-Sep-2024, Manuscript No. mp-25-147465 (R); Published: 12-Feb-2025, DOI: 10.5281/zenodo.16751525
Abstract
Since the beginning of the existence of humanity, plants have been basic resources of life. They play many important roles in nature, such as providing oxygen, medicine, fuel and environmental protection. Since modern medicine began to develop, biologically active compounds from plants have displayed a role as medicine in the fight against pain and disease. In addition to their benefits to human health, it is observed that the focus on phytochemicals is increasing day by day due to many reasons such as the increase in health expenditures, the increase in synthetic drug prices, and the low side effects. Supercritical extraction with CO2 is a technique of Supercritical Fluid Extraction (SFE) which is as a separative technology that uses supercritical liquid solvent for the extraction process. We aimed to determine characteristics of carrot (Daucus carota L. subsp. sativus (Hoffm.) Arcang) performed by Supercritical Liquid CO2 Extraction. Carrot (Daucus carota L. subsp. sativus (Hoffm.) Arcang) samples were obtained from the Konya region (central Turkey) and the samples were recorded in the Herbarium of Necmettin Erbakan University Department of Biology (S. Doğu 4048). Results were effected with different products of the plant such as the juice, essential oil and extract and these results are partly affected by the phytochemical differences in the structure of the plants. Since these findings are given for different extraction methods for carrots, our results can be an example of a different extraction technique and novel application. There are previous studies about carrots but those which have subjected carrots to a supercritical carbon dioxide extraction technique are very limited. Our results has some similarities to previous studies, which are referenced and described here, but we have new findings in our study. The results are compared with different plant materials and other extraction methods of carrots.
Keywords
Daucus carota; Supercritical CO2 extraction; β carotenes
Introduction
Since the beginning of the existence of humanity, plants have been basic resources of life. They play many important roles in nature, such as providing oxygen, medicine, fuel and environmental protection. Since modern medicine began to develop, biologically active compounds from plants have displayed a role as medicine in the fight against pain and disease. In addition, interest in phytochemical components and their chemical structures has accelerated in the last decade. In addition to their benefits to human health, it is observed that the focus on phytochemicals is increasing day by day due to many reasons such as the increase in health expenditures, the increase in synthetic drug prices, and the low side effects.
All around the world, carrot (Daucus carota L. subsp. sativus (Hoffm.) Arcang) contributes significantly to the overall agricultural income. Also, it is remarkable because it is good for the gut and has a high rate of pro-vitamin A and some nutraceuticals. Recent investigations reported that carrot has good efficacy and benefits for health (Buttery, et al.,1969) According to the literature, a deficiency of vitamin A is the major reason for blindness in adolescents. Only 100-gram carrot may meet the carotene (daily) demand for the body (Sulaeman et al, 2001). About 600 carotenoids have been described in this subject, besides α and β carotenes are the most important components (Heinonen, et al,. 1990).
Supercritical extraction with CO2 is a technique of Supercritical Fluid Extraction (SFE) which is a separative technology that uses supercritical liquid solvent for the extraction process. Along with the other options, CO2 is used as supercritical fluid. The SFE uses supercritical fluid to meet a vast range of useful properties when compared to the traditional soxhlet. It also eliminates the usage of the organic solvents, as well as reduction of storage, disposal, and environmental concerns. In this process, lipids and waxes (diffusion coefficients) in supercritical fluids are much higher than in liquids, so extraction may act more quickly. Moreover there is no surface tension in supercritical fluids and their viscosity is much lower than liquids; this helps supercritical fluids penetrate tiny pores that liquid cannot reach (Yang and Hu, 2011) . Carbon dioxide (non-toxic) allows SFE at temperatures near room temperature and relatively low pressures (8 MPa-10 MPa) (Rhoda et al., 2013). In a recent study, carrot root oil (SCO-CO2) extraction was qualified and compared to commercial carrot oil and a virgin olive oil. SCO displayed much more higher contents of carotenes, waxes, phytosterols, sesquiterpene, monoterpene and phenolics volatiles (Ranalli et al., 2004).
At the study, we aimed to determine characteristics of carrot (Daucus carota L. subsp. sativus (Hoffm.) Arcang) performed by Supercritical Liquid CO2 Extraction.
Materials and Methods
Materials
In August 2023, Carrot (Daucus carota L. subsp. sativus (Hoffm.) Arcang) samples were obtained from the Konya region (central Turkey) and the samples were recorded in the Herbarium of Necmettin Erbakan University Department of Biology (S. DoÄ?u 4048). Carrot pieces were dried in the open air (protected from the sunlight). The pieces were ground in a blender for 60 seconds (model EMR-0-01, 28000 rpm, Emir, Istanbul, Turkey) and then performed to supercritical CO2 extraction. The ground particle size of the carrot was 0.55 mm on average. Commercial CO2 with 99% purity was provided by Doganoksigen (DoÄ?anoksijen Ltd, Türkiye). Carnosic acid and carnosol (standard compounds) were used for chemical analysis (Sigma Chemical Co. (St. Louis, USA). Chemicals were at the analytical reagent grade.
Extractions with supercritical carbon dioxide (SC-CO2) were performed in the P-25 35L Supercritical CO2 Extractor System device (Nantong Borisbang Industrial Technology Co., Ltd, BIT HUAAN). The device consists of a high-pressure vessel (25 L) and high-performance cyclonic separator (8 L) operating at to 300 bar at 45 C0. Detailed description of SFE apparatus and experimental procedure were given in a previous study (Papamichail et al., 2000). The amount of approximately 270 grams of ground carrot pieces was placed between two layers of glass beads to reduce dead space in the extractor vessel and allow uniform distribution of the solvent flow in all experiments at 300 bar, equal to 5°C. The particle size of the carrot pieces was low enough (about 200 μm) to ensure well lysis of the plant cells and therefore access to the existing content without solvent. According to the previous studies, the overall efficiency remains almost the same for particle sizes were less than 0.5 mm (Brunner et al., 2013).
Methods
Supercritical carbon dioxide (CO2) extraction: Extraction with supercritical CO2 (experiment A and the final step of experiments I, II, and III) was carried out in a semi-batch Autoclave Engineers Screening System (Autoclave Engineer, Erie, Pennsylvania, USA). According to the study results, all supercritical CO2 extraction experiments were carried out isothermally at 50 C0 and 150 bar, which is the optimal condition for diterpene selectivity (Glisic et al., 2008). Various SFE’s (re-extraction process) were carried out using 30 grams of ground plant material as well as the specified amount of extract in the case of experiments I and II or plant residue in experiment III. Moreover, extraction was performed at 300 bar and 50°C to obtain the total extract with supercritical CO2 and for further comparison with the results of other experiments performed in the presented study (Slinkard and Singleton, 1977). The CO2 flow rate was 0.4 kg in all experiments. Different amounts of extract (fractions) were collected during extraction, and changes in the chemical composition of the extract according to CO2 consumption and pressure were observed along the application. The standard deviation of the measured yield (triplicate procedure) was 2.3% for all experiments. All yield and composition calculations were made on a moisture basis (13.6% moisture by mass). The resulting extracts were also stored in a closed vial at 4°C which was prepared for GC-MS analyses.
Total phenolic quantification: Total phenolic amounts of Daucus carota L. supercritical CO2 extract were measured as equivalent to gallic acid via FCR (Folin Ciocalteu phenol reagent). Solutions containing 1 mg of sample were completed to 4.6 mL with ultrapure water and 100 mL FCR and 3 min were added to this mixture. Then 300 μL of 2% Na2CO3 solution was added. After the mixture was waving for 2 hours at the room temperature, the absorbances (A) were read at the 760 nm (Abdel-Hameed et al., 2012). The total phenolic amounts of the extract were determined with the equation in 2.1. taken from the standard gallic acid chart:
A=0.0123 [ gallic acid (μg) ]- 0.0155, ( r2, 0.9931 ) (2.1.)
Total flavonoid quantification: Daucus carota L. subsp. sativus (Hoffm.) Arcang supercritical CO2 extract was predicted as equivalent to quercetin by the aluminum nitrate method (Moreno et al., 2000). For this purpose, solutions containing 1 mg of sample were made up to 4.8 mL with ethanol, and 100 μL of 1 M potassium acetate and 100 μL of 10% aluminum nitrate solution were added to this mixture. After waiting at room temperature (40 minute), the absorbances were read at the 415 nm. The total flavonoid amounts of the extracts were measured with the equation in 2.2. taken from the standard quercetin graph:
A=0.0156 [ quercetin (μg) ]-0.0112 ( r2, 0.9985 ) (2.2.)
LC-MS/MS analyzes of phenolic compounds of carrot (Daucus carota): The amounts of phytochemical components (μg-metabolites/g plantae) in the carrot (D. carota) samples were calculated with the help of calibration lines drawn between the peak areas and substance concentrations determined as a result of LC-ESI-MS/MS analyses (Pferschy-Wenzig et al., 2009) As shown in fig. 1, the LC-ESI-MS chromatogram of the standards were used for the chromatographic analysis.
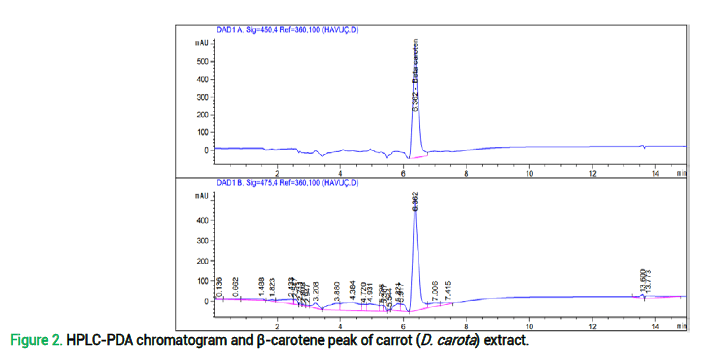
HPLC-PDA analyzes of β-caroten of carrot (Daucus carota): The amount of β-carotene (ppm-β-carotene/g plant) found in the carrot (D. carota) samples was calculated with the help of calibration lines drawn between the peak areas determined as a result of HPLC analyzes and the substance concentrations (Mangunsong et al., 2021). According to the analysis results, the β-carotene peak obtained is shown in the device chromatogram and the beta carotene amount of D. carota extract in ppm is given in fig 1.
Figure 1. Effect of X-Ray dosage on plant growth response.
Antimicrobial activity: To determine the antimicrobial activities of carrot extracts, the broth microdilution method which was developed by (David et al., 2021), was used in the study. Antimicrobial testing was carried out by determining the Minimum Ä°nhibitory Concentration (MIC) values of different carrot extracts against gram-positive, gram-negative bacterial strains and fungi. Bacterial suspensions were adjusted to 0.5 McFarland standard turbidity (108 CFU/ml). Finally, these suspensions which used as inoculums were prepared by diluting fresh cultures to McFarland density of 0.5 at 105 CFU/ml. Müeller Hinton broth (100 μl) medium was dispensed into each well of a 96 well microplate (Keskinkaya et al.,2020). The carrot extract solution, initially prepared at a concentration of 25 mg/ml, was added to the first wells of the microplates and a two-fold dilution of the extract (6.25 mg/mL-0.0030 mg/mL) was made by distributing the solutions to the remaining wells. Then, 100 μl of culture suspension was inoculated into each well. Additionally, DMSO was used as negative control and Gentamicin was used as positive control. Finally, the plates were incubated at 37° C for 18 hours-24 hours. At the end of the incubation period, 20 μl of 2,3,5-triphenyltetrazolium chloride was added to the wells and then incubated for another 30 minutes. The last well on the plates, where there was no visible growth and was not pink or red color.
Results and Discussion
Total phenolic (TPC) and flavonoid (TFC) contents
The total phenolic content value was found as 83.80 μg GAEs/mg ± 0.27 μg GAEs/mg extract and total flavonoid content was 26.49 μg QEs/mg ± 0.34 μg QEs/mg extract (Tab. 1). (Moulick et al. 2023) compared TPC and TFC values of turmeric, beetroot and carrot. TPC values were 200.99 mg GAE/g, 20.95 mg GAE/g, 12.96 mg GAE/g and TFC values were 70.53 mg QE/g, 7.91 mg QE/g, 7.31 mg QE/g extract for turmeric carrot and beetroot respectively. Another study showed TPC value aqueous extract of carrot was 9.59 mg GAE/g while the methanol extracts were changing between 55.10-66.33 and ethanol extracts were changing between 47.44 mg GAE/g -25.30 mg GAE/g depending on the solvents’ density. The same study evaluated the TFC value aqueous extract of carrot at 15.75 mgCE/g and the results were varying between 45.89 mgCE/g -39.08 mgCE/g for methanol extracts and 48.25 mgCE/g-47.83 mgCE/g for ethanol extracts (Mohammed et al., 2022, Anjani et al. 2022) also reported total phenolics and total flavonoids as 89,30 mg and 4.50 mg respectively. The study of (Yusuf et al. 2021) determined total phenolic contents of carrot between 7.3 mg/100 gram FW-224.4 mg/100 gram FW. Total phenolic and flavonoid content of the plants are important to be able to determine potential health benefits due to their antioxidant and antimicrobial properties. However the results can vary related to different factors such as carrot variety, harvesting time, geographical conditions and the part of the plant which has been studied.
| TPC | TFC | |
|---|---|---|
| Extracts | (µg GAEs/mg extractb) | (µg QEs/mg extractc) |
| D. carota | 83.80 ± 0.27 | 26.49 ± 0.34 |
aThe results are given as a mean ± SD of three parallel measurements.
bGAEs, gallic acid equivalent, y=0,0123 x-0,0155 r2=0,9931
cQEs, quercetin equivalent, y=0,0156 x-0,0112 r2=0,9985
Table 1. Total phenolic (TPC) and flavonoid (TFC) contents of D. carota supercritical extraction.
LC- MS/MS and β-carotene analyzes
Various studies focused on different parts of D. carota for the main chemical substances. In our study main components were found in the order of luteolin>coumarin>vanillin>caffein (Fig. 1 and Tab. 2.) A study focused on carrot oil mentioned the main constituents of edible carrot oil as carotol and daucol (Ozcan and Chalchat, 2007), other studies mentioned methylisoeugenol, β-bisabolene, β-asarone (Saad et al., 1995), α-pinene and carotol (Sieniawska et al., 2016), chlorogenic acid, ferulic, caffeic and p-coumaric acids (Arscott and Tanumihardjo, 2010), quercetin, kaempferol and luteolin (Bahorun et al., 2004) as the main components. A recent study (Tlahig et al., 2023) reported quinic acid as the main constituent in different landraces of carrot extracts such as Sidi sallem, Sagui, Arram, El grine, Boughrara and p-coumaric acid and trans cinnamic acid which the common component also determined in our study. (Keser et al. 2020) determined feruloylquinic acid, 3/5 caffeoylquinic acid, di caffeic acid derivative with GC-MS and LC-MS/MS in fresh and microwave dried carrot samples similar to the caffeine component of our study. The amount of β-carotene was determined as 38.37 mg/g extract in this study by using HPLC-PDA Chromatogram (146 mg of extract was dissolved in 6 ml ethylacetate) (Fig. 2). (Anjani et al. 2022) mentioned β-carotene amount of carrot as 8.21 mg/100 g, (Tlahig et al., 2023) determined β-carotene for four different landraces of carrots’ juice between 1255.919 mg/kg-2551.808 mg/kg. Since these results are given for different extraction methods for carrots, our results can be an example for a different extraction technique and novel application.
Figure 2. HPLC-PDA chromatogram and β-carotene peak of carrot (D. carota) extract.
The phase «Beginning of fruiting» in plants was observed on 99 days-112 days from the time of seed sowing. Such a trend was characteristic of the Novichok, Tayana and Lagidny varieties. From the application of Micofrend at a dose of 0.5 or 1.0 l/1000 units of seedlings, the «beginning of fruiting» phase for the Novichok variety began the fastest. In this variant, the beginning of fruiting was observed 99-101 days after sowing the seeds. Other variants of the experiment were characterized by the fact that the beginning of fruiting was observed a little later than the specified variant, but earlier than the plants of the control variant. A longer period of the beginning of fruiting was established in the control version, where mycorrhizal preparations were not used during the cultivation of the Tayana variety 118 days.
The treatment of seedlings with mycorrhizal preparations had a positive effect on the establishment of plants in the open ground, the formation of fruits and the beginning of their technical ripeness due to the positive effect of the fungi Glomus VS, Trichoderma harzianum; microorganisms Streptomyces sp., Pseudomonas fluorescen and bacteria Bacillius megaterium var. phosphaticum, Bacillus subtilis, Bacillus muciloginosus, Enterobacter sp. Tomato fruits were characterized by typical varietal color, shape, weight, and fruit diameter (Tab. 2).
| Compound | RT | Final Concentration (µg/ml) |
|---|---|---|
| Shikimic acid | 1,344 | ND |
| Gallic acid | 3,159 | ND |
| Protocatechuic acid | 5,021 | ND |
| Epigallocatechin | 6,799 | ND |
| Catechin | 7,021 | ND |
| Chlorogenic acid | 7,592 | ND |
| Hydroxybenzaldehyde | 7,767 | ND |
| Vanillic acid | 7,719 | ND |
| Caffeic acid | 7,867 | ND |
| Syringic acid | 8,476 | ND |
| Caffein | 8,615 | 0,0083 |
| Vanillin | 9,014 | 0,0431 |
| Polydatin | 9,201 | ND |
| O-coumaric acid | 9,417 | ND |
| Salicylic acid | 9,590 | ND |
| Taxifolin | 9,733 | ND |
| Resveratrol | 9,976 | ND |
| Trans-ferulic acid | 10,299 | ND |
| Sinapic acid | 10,307 | ND |
| Scutellarin | 11,250 | ND |
| P-coumaric acid | 11,505 | ND |
| Coumarin | 11,392 | 0,1624 |
| Protocatechuic ethyl ester | 11,747 | ND |
| Hesperidin | 11,804 | ND |
| Isoquercitrin | 12,000 | ND |
| Rutin | 12,305 | ND |
| Quarcetin-3-ksilozid | 12,003 | ND |
| Kaempferol-3-glucoside | 13,249 | ND |
| Fisetin | 13,301 | ND |
| Baicalin | 13,594 | ND |
| Chrysin | 14,305 | ND |
| Daidzein | 14,221 | ND |
| Trans-cinnamic acid | 14,643 | ND |
| Quercetin | 14,855 | ND |
| Naringenin | 15,109 | ND |
| Hesperetin | 15,688 | ND |
| Morin | 15,819 | ND |
| Kaempferol | 16,363 | ND |
| Baicalein | 17,093 | ND |
| Luteolin | 17,774 | 0,3349 |
| BÄ°OCHANÄ°N A | 17,960 | ND |
| Capsaicin | 18,236 | ND |
| Dihydrocapcaicin | 18,783 | ND |
| Diosgenin | 23,466 | ND |
Table 2. LC-MS/MS results of carrot (D. carota) supercritical extract.
Antimicrobial activity
In our study, D. carota was tested for its antimicrobial activity against Escherchia. coli, Pseudomonas aeruginosa, Klebsiella pneumoniae, methicillin-resistant Staphylococcus aureus (MRSA), Salmonella enteritidis, Sarcina lutea, Bacillus cereus, and Candida albicans. According to the MIC values, D. carota showed antimicrobial effects against B. cereus (0.19 mg/mL), C. albicans (3.12 mg/mL), and methicillin-resistant S.aureus (3.12 mg/mL) (Tab. 3).
| Test Microorganism | D.carota (SFE) | DMSO | Gentamycin MIC values |
|---|---|---|---|
| MIC values | -100% | (µg/ml) | |
| (mg/ml) | |||
| E.coli | - | 25% | 2.44 |
| Pseudomonas aeruginosa | - | 12.50% | 9.76 |
| Klebsiella pneumoniae | - | 12.50% | 2.44 |
| Methicillin Resistant Staphylococcus aureus (MRSA) | 3.12 | 25% | 78.12 |
| Salmonella enteritidis | - | 25% | 4.88 |
| Sarcina lutea | - | 12.50% | 4.88 |
| Bacillus cereus | 0,19 | 12.50% | 2.44 |
| Candida albicans | 3.12 | 12.50% | 9.76 |
Table 3. Antimicrobial properties of D. carota supercritical extract.
According to MIC values (μg/mL), (Glisic et al. 2008) performed carrot fruit supercritical fluid extracts against to the various microorganisms. The results of the study were determined as 0.64 mg/mL for S. aureus and C. albicans, >1.28 mg/mL for E. coli, S. enteritidis, and P.aeruginosa, and 0.08 mg/mL for B. cereus. Another study conducted by (Tavares et al. 2008), it was found that the MIC values of D. carota essential oil against to C. albicans were ranged from 1.25 to 2.5 μL/mL. A recent study reported that inhibitory concentration of carrot pomace against to S. aureus ATCC 25923 and E. coli ATCC 25922 were at levels of 200 mg/mL, 300 mg/mL, 400 mg/mL, 500 mg/mL, and 600 mg/mL (Sabahi et al., 2024). The obtained MIC results showed that 600 mg/mL was the only effective concentration against the tested microorganisms. (Shindia et al. 2024) tested the antibacterial activity of carrot peel HCl-ethanol extracts against to the gram-positive (B. cereus, S. aureus) and gram-negative (S. typhi, E. coli) bacteria. At the study, MIC values were measured as 50 μg/mL for B. cereus, 20 μg/mL for S. aureus, 25 μg/mL for S. typhi, and 25 μg/mL for E. coli. MIC values of D. carota methanolic peel extract were tested against to E. coli and S. aureus, and determined to be as 150 mg/mL and 200 mg/mL, respectively (Bello et al., 2019). In another study, the MIC values of carrot juice essential oil processed were evaluated. From the results, it was indicated that the MIC values for S. aureus were ranged from 160 mm to 320 mm for 40 μg/disc, while the values for E. coli were 320 mm for all treatments, and for S. enteritidis, the values ranged from 160 mm to 640 mm (Ma et al., 2015). These results were compatible with our study for the inhibition of S. aureus. Some of our results demonstrated similar findings on the comparable microorganisms, but differences in results may occur due to variations in products or plant parts used, such as juice, pomace, peel, essential oil, or extracts. Additionally, these results may be affected by the phytochemical differences in the plant structures.
Conclusions
In the presented study, carrot (Daucus carota L. subsp. sativus (Hoffm.) Arcang extraction has been performed by using the supercritical CO2 extraction method and its total phenolic and flavonoid quantification, LC-MS/MS analyzes of phenolic compounds, HPLC-PDA analyzes of β-carotene and antimicrobial activity were determined and evaluated. There are previous studies about carrots but those which have subjected carrots to a supercritical carbon dioxide extraction technique are very limited. Our results has some similarities to previous studies, which are referenced and described here, but we have new findings in our study. The results are compared with different plant materials and other extraction methods of carrots.
Conflicts of Interest
The authors declare that they have no conflict of interest in this article.
References
Abdel-Hameed ESS, Bazaid SA, Shohayeb MM, El-Sayed MM, El-Wakil EA. (2012). Phytochemical studies and evaluation of antioxidant, anticancer and antimicrobial properties of Conocarpus erectus L. growing in Taif, Saudi Arabia. Eur J Med Plants. 2:93-112. [Google Scholar] [Crossref]
Anjani G, Ayustaningwarno F, Eviana R. (2022). Critical review on the immunomodulatory activities of carrot’s β-carotene and other bioactive compounds. J Funct Foods. 99:105303. [Google Scholar] [Crossref]
Arscott SA, Tanumihardjo SA. (2010). Carrots of many colors provide basic nutrition and bioavailable phytochemicals acting as a functional food. Compr Rev Food Sci Food Saf. 9:223-239. [Google Scholar] [Crossref]
Bahorun T, Luximonâ?Ramma A, Crozier A, Aruoma OI. (2004). Total phenol, flavonoid, proanthocyanidin and vitamin C levels and antioxidant activities of Mauritian vegetables. J Sci Food Agric. 84:1553-1561. [Google Scholar] [Crossref]
Bello M, Bukuyum YM, Danraka RB. (2019). Antibacterial activity of Daucus carota (carrot) peel extract on some selected clinical isolates from Maryam Abacha Women and Children Hospital, Sokoto. Eur J Pharm Med Res. 6:45-51. [Google Scholar]
Brunner G. (2013). Gas extraction: an introduction to fundamentals of supercritical fluids and the application to separation processes. Springer Sci Bus Media. 4. [Google Scholar]
Buttery RG, Seifert RM, Guadagni DG, Ling LC. (1969). Characterization of some volatile constituents of bell peppers. J Agric Food Chem. 17:1322-1327. [Google Scholar] [Crossref]
David V, Andrea AN, Aleksandr K, Lourdes JA, Eugenia P, Nancy C. (2021). Validation of a method of broth microdilution for the determination of antibacterial activity of essential oils. BMC Res Notes. 14:1-7. [Google Scholar] [Crossref]
Glisic S, Smelcerovic A, Zuehlke S, Spiteller M, Skala D. (2008). Extraction of hyperforin and adhyperforin from St. John's Wort (Hypericum perforatum L.) by supercritical carbon dioxide. J Supercrit Fluids. 45:332-337. [Google Scholar] [Crossref]
Heinonen MI. (1990). Carotenoids and provitamin A activity of carrot (Daucus carota L) cultivar. J Agric Food Chem. 38:609-612. [Google Scholar] [Crossref]
Keser D, Guclu G, Kelebek H, Keskin M, Soysal Y, Sekerli YE, Selli S. (2020). Characterization of aroma and phenolic composition of carrot (Daucus carota ‘Nantes’) powders obtained from intermittent microwave drying using GC–MS and LC–MS/MS. Food Bioprod Process. 350-359. [Google Scholar] [Crossref]
Ma T, Luo J, Tian C, Sun X, Quan M, Zheng C, Zhan J. (2015). Influence of technical processing units on chemical composition and antimicrobial activity of carrot (Daucus carrot L.) juice essential oil. Food Chem. 170:394-400. [Google Scholar] [Crossref]
Mangunsong S, Taswin M, Natarajan SB. (2021). Determine β-carotene in carrot (Daucus carota L.) by using HPLC and GC-MS. Agric Food Sci. 10:21-27. [Google Scholar]
Mohammed EA, Abdalla IG, Alfawaz MA, Mohammed MA, Al Maiman SA. (2022). Effects of extraction solvents on the total phenolic content, total flavonoid content, and antioxidant activity in the aerial part of root vegetables. Agric. 12:1820. [Google Scholar] [Crossref]
Moreno MIN, Isla MI, Sampietro AR, Vattuone MA. (2000). Comparison of the free radical-scavenging activity of propolis from several regions of Argentina. J Ethnopharmacol. 71:109-114. [Google Scholar] [Crossref]
Moulick SP, Jahan F, Islam MB, Al Bashera M, Hasan MS, Islam MJ, Bhuiyan MNH. (2023). Nutritional characteristics and antiradical activity of turmeric (Curcuma longa L.), beetroot (Beta vulgaris L.), and carrot (Daucus carota L.) grown in Bangladesh. Heliyon. 9:11. [Google Scholar] [Crossref]
Özcan MM, Chalchat JC. (2007). Chemical composition of carrot seeds (Daucus carota L.) cultivated in Turkey: characterization of the seed oil and essential oil. Grasas Aceites. 58:359-365. [Google Scholar] [Crossref]
Papamichail I, Louli V, Magoulas K. (2000). Supercritical fluid extraction of celery seed oil. J Supercrit Fluids. 18:213-226. [Google Scholar] [Crossref]
Pferschy-Wenzig EM, Getzinger V, Kunert O, Woelkart K, Zahrl J, Bauer R. (2009). Determination of falcarinol in carrot (Daucus carota L.) genotypes using liquid chromatography/mass spectrometry. Food Chem. 114:1083-1090. [Google Scholar] [Crossref]
Ranalli A, Contento S, Lucera L, Pavone G, Di Giacomo G. (2004). Characterization of carrot root oil arising from supercritical fluid carbon dioxide extraction. J Agric Food Chem. 52:4795-4801. [Google Scholar] [Crossref]
Rhoda BLeron, Alvin Caparanga, Meng-Hui Li. (2013). Carbon dioxide solubility in a deep eutectic solvent based on choline chloride and urea at T=303.15-343.15K and moderate pressures. J Taiwan Inst Chem Eng. 44:879-885. [Google Scholar] [Crossref]
Saad HA, El-Sharkawy SH, Halim AF. (1995). Essential oils of Daucus carota ssp. maximus. Pharm Acta Helv. 70:79-84. [Google Scholar] [Crossref]
Sabahi S, Abbasi A, Mortazavi A. (2024). Phenolic components from carrot (Daucus carota L.) pomace: optimizing the extraction and assessing its potential antioxidant and antimicrobial activities. Heliyon. [Google Scholar] [Crossref]
Shindia A, Abdel-Shafi S, Atef A, Osman A, Sitohy B, Sitohy S. (2024). Antibacterial activity of carrot peel HCl-ethanol extracts and its potential application in meat preservation. LWT. 207:116638. [Google Scholar] [Crossref]
Sieniawska E, Å?wiÄ?tek Å, Rajtar B, KozioÅ? E, Polz-Dacewicz M, Skalicka-Woźniak K. (2016). Carrot seed essential oil-source of carotol and cytotoxicity study. Ind Crops Prod. 92:109-115. [Google Scholar] [Crossref]
Slinkard K, Singleton VL. (1997). Total phenol analyses: automation and comparison with manual methods. Am J Enol Viticult. 28:49-55. [Google Scholar] [Crossref]
Sulaeman A, Keeler L, Taylor SL, Giraud DW, Driskell JA. (2001). Carotenoid content, physicochemical, and sensory qualities of deep-fried carrot chips as affected by dehydration/rehydration, antioxidant, and fermentation. J Agric Food Chem. 49:3253-3261. [Google Scholar] [Crossref]
Tavares AC, Gonçalves MJ, Cavaleiro C, Cruz MT, Lopes MC, Canhoto J, Salgueiro LR. (2008). Essential oil of Daucus carota subsp. halophilus: composition, antifungal activity and cytotoxicity. J Ethnopharmacol. 119:129-134. [Google Scholar] [Crossref]
Tlahig S, Mohamed A, Yahia LB, Hamrouni N, Bouhamda T, Mabrouk M, Loumerem M. (2023). Root pigmentation determines phytochemical, mineral, antioxidant and organoleptic attributes of Tunisian southern coastal carrot (Daucus carota subsp. sativus) landraces. Scientia Horticulturae. 321:112289. [Google Scholar] [Crossref]
Yang Y, Hu B. (2014). Bio-based chemicals from biorefining: lipid and wax conversion and utilization. Adv Biorefineries. 693-720. [Google Scholar] [Crossref]
Yin S, Niu L, Shibata M, Liu Y, Hagiwara T. (2022). Optimization of fucoxanthin extraction obtained from natural by-products from Undaria pinnatifida stem using supercritical CO2 extraction method. Front Nutr. 9:981176. [Google Scholar] [Crossref]
Yusuf E, Tkacz K, Turkiewicz IP, WojdyÅ?o A, Nowicka P. (2021). Analysis of chemical compounds’ content in different varieties of carrots, including qualification and quantification of sugars, organic acids, minerals, and bioactive compounds by UPLC. Eur Food Res Technol. 247:3053-3062. [Google Scholar] [Crossref]