Research Article - Modern Phytomorphology ( 2025) Volume 0, Issue 0
A meta-analysis of management of elevated intracranial pressure in traumatic brain injury: Hypertonic saline vs. Mannitol
Mohamed S. Imam1*, Abdullah Mahammed Jaber Almalki2, Faisal Saleh Saeed Alswat2, Atheer Zayed Safar Alotaibi2, Nardeen Awad Saed Algethami2, Shoog Hameed Mohammad Altalhi2, Amal Ali Mohammed Alshehri3, Rahaf Naif Alhashbari4, Randa Matuq Aljuaid5, Wejdan Muhsen Alsufyane6 and Ibtisam Eidhah Althubyani72College of Pharmacy, Taif University, Taif 21944, Saudi Arabia
3College of Pharmacy, King Khalid University, Abha 62529, Saudi Arabia
4Ibrand Pharmacy, Taif, Saudi Arabia
5Maaly Alsehah Pharmacy, Taif, Saudi Arabia
6Ghodaf Pharmacy,Taif, Saudi Arabia
7Shams Pharmacy,Taif, Saudi Arabia
Mohamed S. Imam, Department of Pharmacy, AlNahda College of Pharmacy and Medical Sciences, Riyadh, Kingdom of Saudi Arabia, Email: imammohamed311@gmail.com
Received: 18-Aug-2025, Manuscript No. mp-25-170056; , Pre QC No. mp-25-170056 (PQ); Editor assigned: 20-Aug-2025, Pre QC No. mp-25-170056 (PQ); Reviewed: 09-Mar-2025, QC No. mp-25-170056; Revised: 05-Sep-2025, Manuscript No. mp-25-170056 (R); Published: 17-Sep-2025, DOI: 10.5281/zenodo.17085329
Abstract
Background and objectives: We conducted a meta-analysis to evaluate the comparative efficacy of hypertonic saline (HS) versus mannitol in the management of elevated Intracranial Pressure (ICP) among patients with Traumatic Brain Injury (TBI).
Materials and methods: A systematic literature search up to February 2025 identified 18 studies including 1,828 patients with TBI. The analysis compared the effects of HS and mannitol on elevated ICP control. Odds Ratios (ORs) and Mean Differences (MDs) with 95% Confidence Intervals (CIs) were calculated using dichotomous or continuous approaches, applying either fixed- or random-effects models.
Results: Compared with mannitol, HS was associated with a significantly lower treatment failure rate (OR 0.38; 95% CI 0.15-0.98; p=0.04), higher cerebral perfusion pressure at 30-60 minutes post-infusion (MD 5.25; 95% CI 3.59-6.91; p<0.001), and lower ICP at 30-60 minutes (MD-1.12; 95% CI -2.11 to -0.12; p=0.03). However, HS did not significantly affect mortality, functional outcomes, cerebral perfusion pressure at 90-120 minutes, ICP at 90-120 minutes, or the daily duration of elevated ICP.
Conclusion: In patients with TBI, HS was superior to mannitol in reducing treatment failure, improving cerebral perfusion pressure, and lowering ICP within 30-60 minutes of administration. However, no significant differences were observed in mortality, long-term functional outcomes, or ICP control beyond 90 minutes. These findings suggest HS may offer short-term hemodynamic advantages over mannitol, but larger, high-quality trials are required to confirm its long-term clinical benefit.
Keywords
Mannitol, Hypertonic saline, Cerebral perfusion pressure, Intracranial pressure, Traumatic brain injury, Treatment outcome, Stress physiology, Morphological adaptations, Comparative anatomy, Plant morphology, Systematic botany
Introduction
Traumatic Brain Injury (TBI) represents one of the most significant contributors to fatalities and chronic impairments worldwide. Epidemiological data reveal that European populations experience over 2,000 TBI cases per million people each year. A hallmark of severe TBI cases is the development of elevated Intracranial Pressure (ICP), which frequently stems from cerebral edema a pathological buildup of fluid within brain tissue (Graham, et al. 1978, Marion, et al. 1991, Bouma, et al. 1991, Katayama, 1990). Clinicians prioritize ICP control in TBI protocols due to its well-documented association with elevated mortality risks and diminished recovery of neurological or physical function (Scalfani, et al. 2012). First-line interventions outlined in clinical guidelines involve non-surgical methods, such as positioning patients in a semi-recumbent posture, diverting Cerebrospinal Fluid (CSF) via catheterization, and pharmacological therapies including sedatives (e.g., propofol) and hyperosmolar agents. Surgical interventions like decompressive craniectomy are reserved for cases unresponsive to these measures (Firsching, et al. 2017, Carney, et al. 2017, Kochanek, et al. 2019). In neurocritical care, hyperosmolar agents such as mannitol and hypertonic saline are utilized to counteract cerebral edema, preserve Cerebral Perfusion Pressure (CPP), and enhance cerebral hemodynamics by modulating blood flow dynamics.
The application of osmotherapy for cerebral edema is undergirded by a fundamental biological strategy shared with botanical organisms. Faced with environmental extremes, plant systems have evolved sophisticated mechanisms to achieve osmotic equilibrium, safeguarding cellular architecture and viability. A central tactic involves the production and amassment of compatible osmolytes small organic molecules that adjust intracellular volume while preserving metabolic function (Chen, et al. 2002). In this clinical context, mannitol represents a unique example of a plant-derived osmolyte with direct therapeutic application. This hexitol is not merely a pharmaceutical agent but also a principal photosynthate, a reserve carbon source, and a vital osmoprotective compound synthesized in diverse plant species such as olives, celery, and marine algae (Loescher, et al. 1987, Zhifang, et al. 2003). Its natural role in protecting plant cells against osmotic stress provides a strong phytophysiological rationale for its pharmacological use in mitigating cerebral edema. By highlighting mannitol’s dual identity as both a phytochemical and a neurocritical care, this study establishes a bridge between plant-based pharmaceutical research and clinical medicine.
Beyond its clinical effects, mannitol’s biological and chemical origins provide additional context for its role in ICP management. Mannitol can be synthesized from mannose through chemical reduction (e.g., with sodium borohydride) or catalytic hydrogenation, which introduces a hydrogen atom across the carbonyl group, forming a sugar alcohol (Saha, et al. 2011). In plants, mannitol biosynthesis proceeds via enzymatic pathways involving NADPH-mannose-6-phosphate reductase (M6PR). Specifically, (1) mannose is first phosphorylated to mannose-6-phosphate, (2) M6PR reduces this intermediate to mannitol-1-phosphate, and (3) a phosphatase removes the phosphate group to yield mannitol. This biosynthetic process is influenced by light availability and plant developmental stages (Meena, et al. 2015).
However, systemic effects of osmotic agents, such as osmotic diuresis or transient hypernatremia, may inadvertently alter cerebrovascular dynamics, complicating their therapeutic role (Zhang, et al. 2019). Clinical studies strongly endorse the administration of hyperosmolar therapies for managing elevated Intracranial Pressure (ICP) in Traumatic Brain Injury (TBI) patients. However, ongoing controversy surrounds the identification of a preferred therapeutic agent among available options (Firsching, et al. 2017, Carney, et al. 2017, Kochanek, et al. 2019). Current therapeutic protocols employ two primary osmotic agents, Hypertonic Saline (HS) and mannitol both of which are integral to modern neurocritical care (Shi, et al. 2020, Gu, et al. 2019, Schwimmbeck, et al. 2021). While their ability to reduce ICP is well-documented, comparative studies have yet to establish a definitive therapeutic advantage for either agent (Soustiel, et al. 2006). Clinicians frequently favor mannitol despite the absence of standardized approval frameworks guiding its application in ICP management. Preclinical investigations, including animal models, propose potential benefits of HS, such as reduced neuroinflammatory responses, enhanced ICP stabilization, and improved cerebral oxygenation. However, these findings have not been reliably replicated in randomized human trials, highlighting translational discrepancies between experimental and clinical outcomes (Anestesiologica, 2015). Existing meta-analyses focus predominantly on surrogate markers like ICP reduction rather than patient-centered outcomes (e.g., mortality, functional recovery), with most analyses reporting no statistically meaningful disparities between HS and mannitol (Shi, et al. 2020, Gu, et al. 2019, Schwimmbeck, et al. 2021). Consensus guidelines emphasize the inconclusive evidence base for prioritizing one agent over the other, underscoring the need for rigorous, outcome-driven trials (Firsching, et al. 2017, Carney, et al. 2017, Kochanek, et al. 2019). This meta-analysis systematically evaluates the comparative efficacy of HS versus mannitol in reducing elevated ICP among TBI patients, aiming to clarify their roles in clinical practice.
Materials and Methdos
The present investigation adhered to the principles of meta-analysis of epidemiological studies as outlined in established methodological statements and was conducted in accordance with a predefined protocol (Stroup, et al. 2000).
Study selection
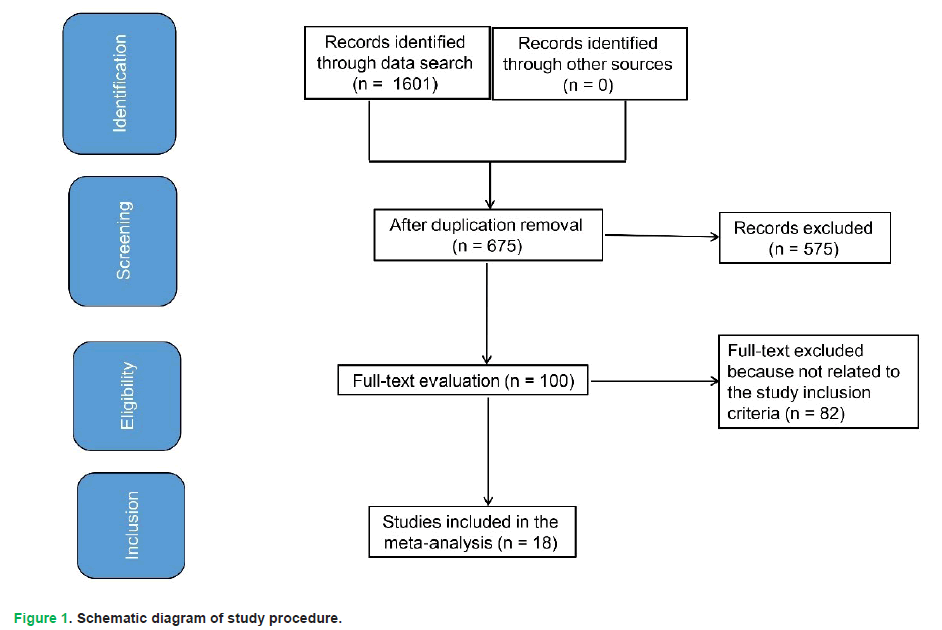
Study criteria encompassed statistical measures of connotation regarding efficacy of HS versus mannitol in managing high ICP in TBI. Inclusion was not limited by size or type of study. Excluded publications comprised review articles, editorials, and studies lacking a measure of association. Fig. 1 illustrates entire study process.
Figure 1: Schematic diagram of study procedure.
Publications were incorporated into meta-analysis upon fulfillment of subsequent inclusion criteria:
i. Investigative designs were limited to prospective Randomized Controlled Trials (RCTs) or retrospective cohort analyses.
ii. Participant eligibility required a confirmed diagnosis of Traumatic Brain Injury (TBI).
iii. Intervention program assessed efficacy of HS against mannitol in managing high ICP in TBI.
iv. Study encompassed comparisons between HS and mannitol.
Criteria for excluding participants from intervention groups were:
i. Research that did not assess the impact of HS versus mannitol in the management of increased ICP in TBI.
ii. Research encompassing ICP management following TBI, excluding impacts of HS and mannitol.
iii. Research that did not concentrate on the impact on comparative outcomes.
Identification
The research protocol was structured around the PICOS framework (population, intervention, comparison, outcomes and study design) to ensure methodological rigor (Higgins, et al. 2003). This framework was operationalized as follows:
• Population (P): Patients diagnosed with Traumatic Brain Injury (TBI).
• Intervention/Exposure (I): Administration of Hypertonic Saline (HS) or mannitol.
• Comparison (C): Relative efficacy of HS versus mannitol in clinical outcomes.
• Outcomes (O): Primary metrics included Treatment Failure (TF), favorable Functional Outcomes (FOs), mortality rates, and changes in Cerebral Perfusion Pressure (CPP) and Intracranial Pressure (ICP).
• Study design (S): All study types were eligible for inclusion to minimize selection bias (Liberati, et al. 2009).
A comprehensive search was conducted in five databases (Embase, PubMed, Cochrane Library, OVID, and Google Scholar) up to February 2025. The search strategy incorporated controlled vocabulary (MeSH terms) and free-text keywords related to HS, mannitol, ICP modulation, TBI, CPP, mortality, and functional recovery (Tab. 1). Retrieved records were imported into EndNote, where duplicates were systematically removed. Titles and abstracts were screened in a two-tier process to exclude studies without direct HS-mannitol comparisons. Full-text reviews were conducted for the remaining studies to extract data aligned with the predefined outcomes. To reinforce the journal’s thematic scope, it is important to note that mannitol was considered not only as a neurocritical care intervention but also as a plant-derived osmolyte, linking this clinical study with phytochemical origins. This dual identity ensures methodological relevance for Modern Phytomorphology, particularly in the context of phytomedicine.
| Database | Search strategy |
|---|---|
| PubMed | #1 "hypertonic Saline"[MeSH Terms] OR "mannitol"[All Fields] OR "intracranial pressure"[All Fields] #2 "traumatic brain injury"[MeSH Terms] OR "hypertonic Saline"[All Fields] OR "Treatment failure"[All Fields] OR "Cerebral perfusion pressure"[All Fields] OR "Favorable outcome"[All Fields] OR "Death"[All Fields] #3 #1 AND #2 |
| Embase | #1 'hypertonic Saline'/exp OR 'mannitol'/exp OR 'intracranial pressure'/exp #2 'traumatic brain injury'/exp OR 'ICBG'/exp OR 'Treatment failure'/exp OR 'Cerebral perfusion pressure'/exp OR 'Favorable outcome'/exp OR 'Death'/exp #3 #1 AND #2 |
| Cochrane library | #1 (hypertonic Saline):ti,ab,kw OR (mannitol):ti,ab,kw OR (intracranial pressure):ti,ab,kw (Word variations have been searched) #2 (traumatic brain injury):ti,ab,kw OR (Treatment failure):ti,ab,kw OR (Cerebral perfusion pressure):ti,ab,kw OR (Favorable outcome):ti,ab,kw OR (Death):ti,ab,kw (Word variations have been searched) #3 #1 AND #2 |
Table 1. Search strategy for each database.
Screening
Data extraction followed a standardized template capturing study identifiers (lead author, year, timeframe, location), design characteristics, cohort demographics, intervention protocols, outcome measures, statistical methods, and comparative efficacy findings (Gupta, et al. 2018). Two reviewers independently screened and coded eligible studies. Disagreements were resolved through discussion, and unresolved conflicts were adjudicated by a senior investigator. To minimize bias, the Cochrane Collaboration’s RoB 2 tool was applied to evaluate methodological rigor in RCTs (RoB, 2020), classifying studies into three categories:
i. Low risdecreased intracranial pressure (ICP) within 30-60 minutes aftelaws or omissions that compromised validity.
Consensus was reached through re-examination of source materials, and final classifications were documented.
Eligibility
The primary outcome was the comparative impact of HS versus mannitol on elevated ICP management in TBI. A summary evaluation of associated clinical outcomes (e.g., mortality, CPP, and functional recovery) was conducted.
Inclusion
Sensitivity analyses were restricted to studies documenting direct HS versus mannitol comparisons. Subclass and sensitivity assessments were undertaken to validate findings.
Statistical analysis
Analytical computations included Odds Ratios (ORs) and Mean Differences (MDs) with 95% Confidence Intervals (CIs), derived using fixed-effect or random-effects models depending on data heterogeneity. Heterogeneity was quantified using the I2 index, with thresholds defined as follows:
• 0%-25%: Minimal heterogeneity
• 26%-50%: Moderate heterogeneity
• >50%: Substantial heterogeneity, warranting a random-effects model to account for inter-study variance (Higgins, et al. 2003).
Subgroup analyses were performed based on predefined outcome categories, with intergroup differences considered statistically significant at p<0.05. Publication bias was evaluated quantitatively using Egger’s regression test (bias inferred at p ≥ 0.05) and qualitatively by funnel plot asymmetry (log-transformed ORs vs. standard errors) (Gupta, et al. 2018).
All statistical analyses were performed using Reviewer Manager 5.3 (The Nordic Cochrane Centre, Cochrane Collaboration). Forest plots and sensitivity analyses were generated to visualize pooled effect estimates.
Results
A total of 1,601 distinct research studies were discovered, of which 18 investigations (conducted between 2003 and 2023) fit the inclusion criteria and were incorporated into this meta-analysis (Vialet, et al. 2003, Harutjunyan, et al. 2005, Mao, et al. 2007, Francony, et al. 2008, Oddo, et al. 2009, Kerwin, et al. 2009, Ichai, et al. 2009, Cottenceau, et al. 2011, Sakellaridis, et al. 2011, Hendoui, et al. 2013, Huang, et al. 2014, Jagannatha, et al. 2016, Du, et al. 2017, Qin, et al. 2018, Patil, et al. 2019, Huang, et al. 2020, Mangat, et al. 2020, Van Veen, et al. 2023). A total of 18 trials encompassed 1,828 participants with TBI at the commencement of research, as shown in Tab. 2. All trials assessed the impact of HS versus mannitol in the treatment of high ICP resulting from TBI. The study included between 20 and 457 patients with traumatic brain damage at its inception.
| Study | Country | Total | Hypertonic saline | Mannitol |
|---|---|---|---|---|
| Vialet, 2003 [20] | France | 20 | 10 | 10 |
| Harutjunyan, 2005 [21] | Germany | 32 | 17 | 15 |
| Mao, 2007 [22] | China | 56 | 28 | 28 |
| Francony, 2008 [23] | USA | 20 | 10 | 10 |
| Oddo, 2009 [24] | USA | 22 | 11 | 11 |
| Kerwin, 2009 [25] | USA | 22 | 11 | 11 |
| Ichai, 2009 [26] | France | 33 | 17 | 16 |
| Cottenceau, 2011 [27] | France | 47 | 22 | 25 |
| Sakellaridis, 2011 [28] | Greece | 64 | 32 | 32 |
| Hendoui, 2013 [29] | Iran | 33 | 23 | 10 |
| Huang, 2014 [30] | India | 238 | 119 | 119 |
| Jagannatha, 2016 [31] | India | 38 | 18 | 20 |
| Du, 2017 [32] | China | 132 | 65 | 67 |
| Qin D, 2018 [33] | China | 48 | 24 | 24 |
| Patil, 2019 [34] | India | 80 | 40 | 40 |
| Huang, 2020 [35] | China | 457 | 236 | 221 |
| Mangat, 2020 [36] | USA | 50 | 25 | 25 |
| van Veen, 2023 [37] | Netherlands | 436 | 287 | 149 |
| Total | 1828 | 995 | 833 |
Table 2. Characteristics of selected studies for meta-analysis.
Analysis of acute-phase outcomes (30-60 minutes post-infusion) revealed distinct advantages for Hypertonic Saline (HS) over mannitol in Traumatic Brain Injury (TBI) cohorts:
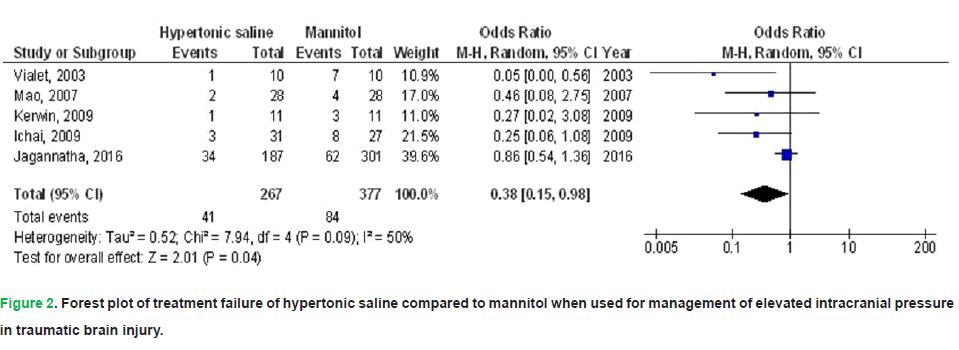
• Treatment Failure (TF): HS was associated with a 62% reduction in TF incidence (OR: 0.38; 95% CI: 0.15-0.98; p=0.04), though moderate heterogeneity was noted across studies (I2=50%).
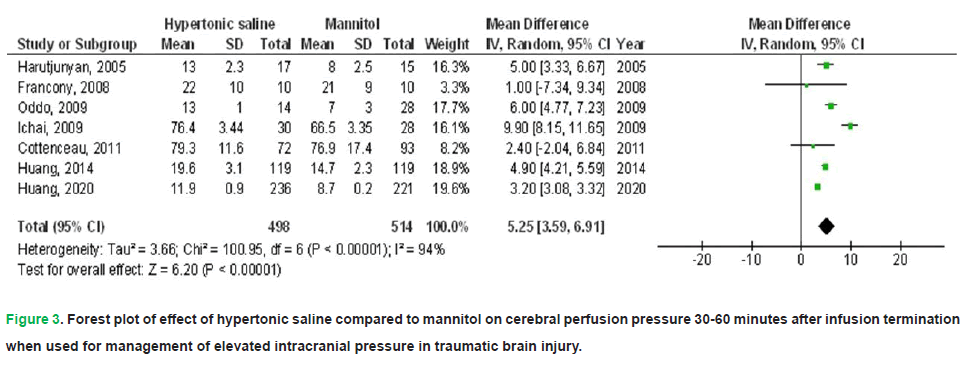
• Cerebral Perfusion Pressure (CPP): A marked increase in CPP of 5.25 mmHg (95% CI: 3.59-6.91; p<0.001) was observed with HS, albeit with substantial interstudy variability (I2=94%).
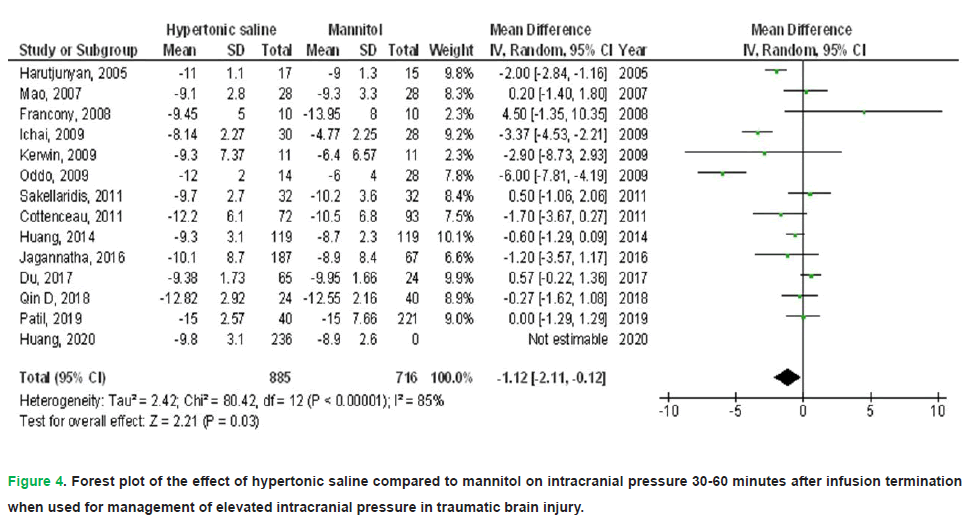
• Intracranial Pressure (ICP): HS administration correlated with a 1.12 mmHg reduction in ICP (95% CI: -2.11–-0.12; p=0.03), despite significant heterogeneity among trials (I2=85%).
Figure 2. Forest plot of treatment failure of hypertonic saline compared to mannitol when used for management of elevated intracranial pressure in traumatic brain injury.
Figure 3: Forest plot of effect of hypertonic saline compared to mannitol on cerebral perfusion pressure 30-60 minutes after infusion termination when used for management of elevated intracranial pressure in traumatic brain injury.
Figure 4: Forest plot of the effect of hypertonic saline compared to mannitol on intracranial pressure 30-60 minutes after infusion termination when used for management of elevated intracranial pressure in traumatic brain injury.
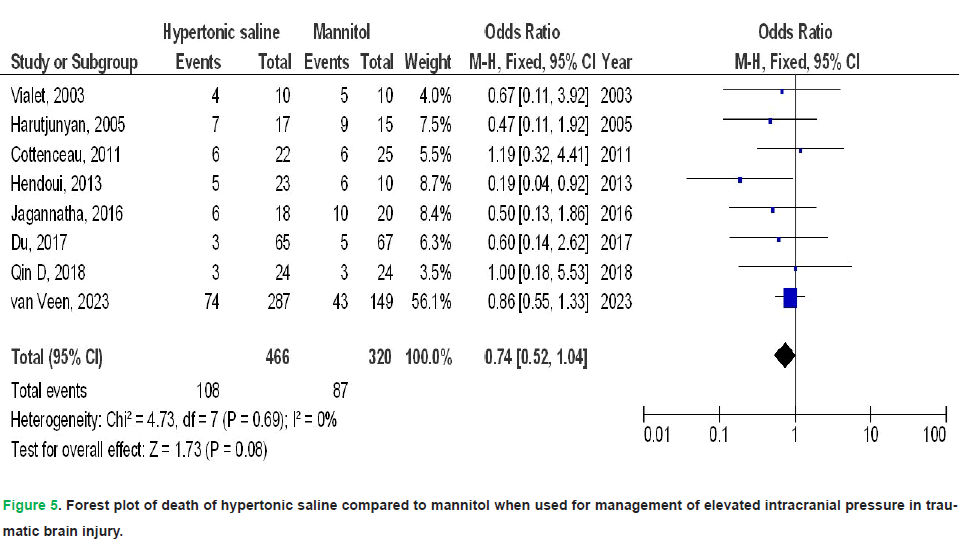
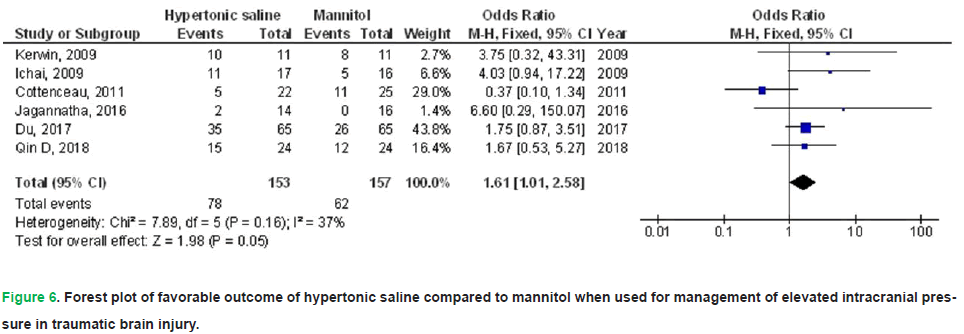
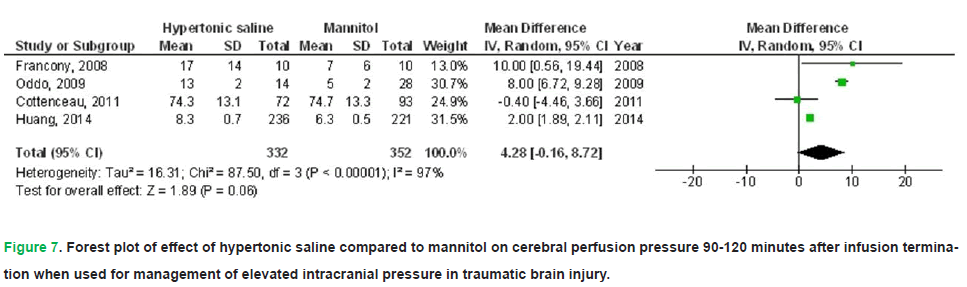
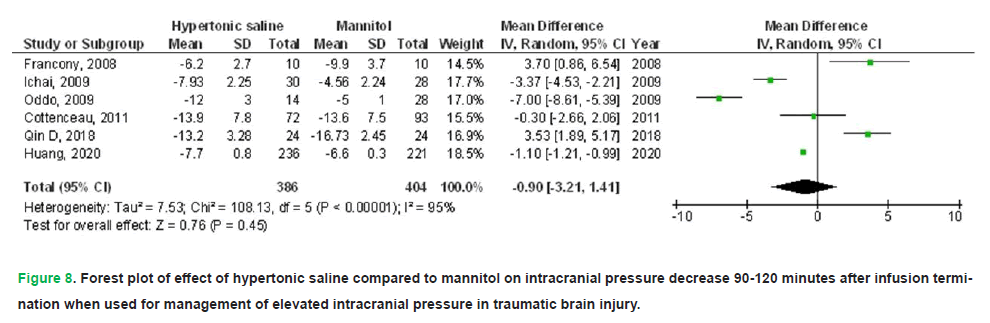
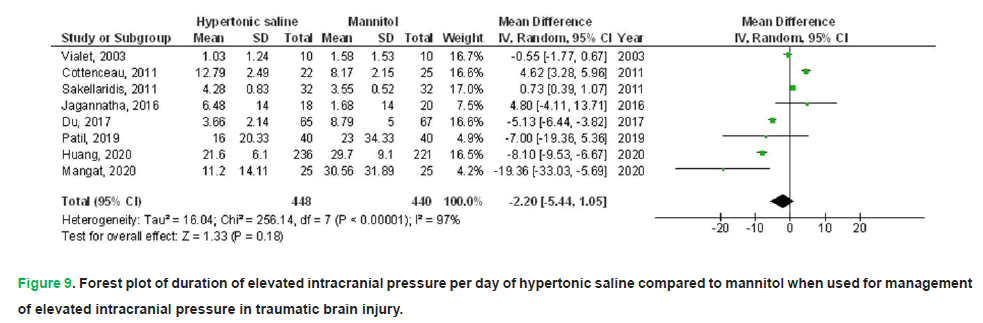
HS exhibited no significant impact on death (OR, 0.74; 95% CI, 0.52-1.04, p=0.08) with no heterogeneity (I2=0%), a FO (OR, 1.61; 95% CI, 1.01-2.58, p=0.05) with low heterogeneity (I2=37%), CPP 90-120 minutes post-infusion termination (MD, 4.28; 95% CI, -0.16-8.72, p=0.06) with high heterogeneity (I2=97%), ICP 90-120 minutes post-infusion termination (MD, -0.90; 95% CI, -3.21-1.41, p=0.45) with high heterogeneity (I2=95%), and duration of elevated ICP per day (MD, 2.20; 95% CI, -5.44-1.05, p=0.18) with high heterogeneity (I2=97%) in comparison to mannitol in individuals with TBI, as illustrated in Figs. 5-9.
Figure 5: Forest plot of death of hypertonic saline compared to mannitol when used for management of elevated intracranial pressure in traumatic brain injury.
Figure 6: Forest plot of favorable outcome of hypertonic saline compared to mannitol when used for management of elevated intracranial pressure in traumatic brain injury.
Figure 7: Forest plot of effect of hypertonic saline compared to mannitol on cerebral perfusion pressure 90-120 minutes after infusion termination when used for management of elevated intracranial pressure in traumatic brain injury.
Figure 8: Forest plot of effect of hypertonic saline compared to mannitol on intracranial pressure decrease 90-120 minutes after infusion termination when used for management of elevated intracranial pressure in traumatic brain injury.
Figure 9: Forest plot of duration of elevated intracranial pressure per day of hypertonic saline compared to mannitol when used for management of elevated intracranial pressure in traumatic brain injury.
Stratified analyses of selected studies that accounted for age, gender, and ethnicity were not conducted, as no research reported or adjusted for these variables. The visual examination of the funnel plot, along with quantitative analysis via the Egger regression test, revealed no indication of publication bias (p=0.87). Nevertheless, the majority of included studies were evaluated as having low methodological quality owing to their limited sample size. All studies exhibited no selective reporting bias, and no articles had incomplete outcome data or selective reporting.
Discussion
This meta-analysis, comprising 18 studies, included 1,828 participants with TBI at baseline (Vialet, et al. 2003, Harutjunyan, et al. 2005, Mao, et al. 2007, Francony, et al. 2008, Oddo, et al. 2009, Kerwin, et al. 2009, Ichai, et al. 2009, Cottenceau, et al. 2011, Sakellaridis, et al. 2011, Hendoui, et al. 2013, Huang, et al. 2014, Jagannatha, et al. 2016, Du, et al. 2017, Qin, et al. 2018, Patil, et al. 2019, Huang, et al. 2020, Mangat, et al. 2020, Van Veen, et al. 2023). In acute analyses (30-60 minutes post-infusion), Hypertonic Saline (HS) showed lower treatment failure, higher CPP, and modestly lower ICP than mannitol. However, by 90-120 minutes post-infusion, these effects attenuated and no consistent between-group differences were observed across mortality, favorable Functional Outcomes (FOs), CPP, ICP, or the daily duration of ICP elevation. Accordingly, the question of whether HS confers durable neuroprotective superiority over mannitol remains unresolved. Potential contributors to this uncertainty include study-level biases (e.g., selection bias, incomplete blinding in early trials such as Vialet 2003) and variability in dosing protocols, co-interventions, and monitoring windows (Vialet, et al. 2003). Interpretation should be cautious, particularly for outcomes reported by few studies (FOs, death, CPP at 90-120 minutes).
The meta-analysis was further constrained by the predominance of studies with limited participant cohorts, notably 14 trials enrolling fewer than 100 individuals each. This restricted sample size raises concerns about statistical power and generalizability of the aggregated findings, underscoring necessity for extra studies to validate these findings and possibly improve reliability of effect assessments, particularly concerning FOs, death, and CPP, which exhibited very low p-values (p=0.05, 0.06, and 0.06). Intracranial hypertension resulting from TBI may lead to several complications, including cerebral ischemia, Cushing’s response, brain displacement, and neurogenic pulmonary edema (Maas, et al. 2017). Elevated Intracranial Pressure (ICP), traditionally classified as exceeding 20 mmHg, has long served as a clinically established threshold for initiating targeted therapies in neurocritical care. Monitoring ICP remains a critical prognostic indicator of neurological decline among Traumatic Brain Injury (TBI) patients, as sustained elevations correlate strongly with adverse functional outcomes (Juul, et al. 2001). Research indicates that when CPP is critically low (<50 mm Hg), ICP serves as an indicator of unFOs, whereas maintaining ICP between 18 and 23 mmHg ensures prolonged stability of CPP (Rangel-Castillo, et al. 2008).
Mannitol has been a long-standing therapeutic agent employed to mitigate raised Intracranial Pressure (ICP), a critical intervention in neurocritical care. Contemporary clinical guidelines suggest its superior efficacy over barbiturates for ICP reduction in TBI patients, reinforcing its role as a first-line osmotherapeutic option (Carney, et al. 2017). Mannitol, however, has side effects such as pulmonary edema, abrupt renal failure, rebound cerebral edema, exacerbation, and arterial hypotension, which leads to a decrease in CPP due to its diuretic properties. Optimal treatment agent for managing ICP should decrease it while maintaining cerebral perfusion (Schwimmbeck, et al. 2021). While Hypertonic Saline (HS) is generally regarded as safe, prolonged infusions targeting serum sodium concentrations of 170 mmol/L have been linked to infrequent but severe complications, including hematological disturbances (neutropenia, thrombocytopenia, anemia), acute kidney injury, and Acute Respiratory Distress Syndrome (ARDS) (Gonda, et al. 2013). HS exerts its therapeutic effects by elevating serum sodium and plasma osmolality; however, supraphysiological levels may induce systemic complications. Excessive sodium accumulation can provoke fluid shifts, contributing to cardiopulmonary compromise (e.g., pulmonary edema, cardiac strain) or metabolic derangements such as hyperchloremic acidosis and coagulopathies (Treib, et al. 1997, Moon and Kramer, 1995). Consequently, hypertonic solutions administered to individuals with impaired cardiac function must be approached with carefulness and accompanied by vigilant cardiac monitoring. Consistent with our findings, intravenous administration of HS enhanced cerebral perfusion, induced a shift in oxygen dissociation curve, so augmenting oxygen delivery, improving brain compliance, and decreasing cerebral edema and ICP (Kempski, et al. 1996). Nevertheless, evidence regarding efficacy of HS in severe TBI is insufficient in demonstrating its benefits in reducing ICP and death rates (Rangel-Castillo, et al. 2008).
The observed time dependence (benefit at 30-60 minutes, dissipation by 90-120 minutes) highlights the importance of infusion kinetics and repeat-dosing strategies. Prior observations that rapid boluses may yield shorter-lived effects, whereas slower infusions (e.g., over 20-30 minutes) could mitigate rebound, suggest protocolized dosing and re-dosing intervals should be standardized in trials (Battison, et al. 2005). Future multicenter RCTs should pre-specify dosing rate, cumulative exposure, re-dosing criteria, rescue thresholds, and uniform timepoints for ICP/CPP assessment, and should stratify by injury severity, lesion phenotype, and multimodal neuromonitoring context. They should also be powered for patient-centered endpoints (mortality and functional outcomes), not just surrogate ICP metrics. Because most included studies did not adjust for age, sex, or ethnicity, future work should prespecify these covariates and explore effect modification.
Mannitol is a plant-derived polyol and compatible osmolyte; plants synthesize mannitol via the mannose-6-phosphate pathway (M6PR → mannitol-1-phosphate → mannitol), while industrial production often employs chemical reduction or catalytic hydrogenation of mannose. This phytochemical clinical nexus aligns with the special issue “Digital Innovations in Plant-based Pharmaceutical Research and Phytomedicine.” Our clinical synthesis provides endpoint benchmarks that can inform digital pipelines, e.g., cheminformatic optimization of plant-derived mannitol production, systems biology models of osmolyte transport, and AI-assisted dosing algorithms that reconcile pharmacokinetics with neuromonitoring signals, thereby translating plant biochemistry into evidence-based neurocritical protocols.
In summary, HS shows short-term physiological advantages over mannitol, but durable clinical superiority is unproven; rigorous, adequately powered trials with standardized dosing and patient-centered endpoints are needed. Given mannitol’s botanical origin and dual identity as a phytochemical and therapeutic, integrating digital phytomedicine frameworks with clinical trial design represents a timely direction for this field.
Conclusion
Hypertonic Saline (HS) demonstrated a substantially reduced treatment failure rate, increased Cerebral Perfusion Pressure (CPP), and decreased Intracranial Pressure (ICP) within 30-60 minutes after infusion cessation compared to mannitol in patients with Traumatic Brain Injury (TBI). However, beyond 90-120 minutes, HS showed no significant difference from mannitol in terms of mortality, Functional Outcomes (FOs), CPP, ICP, or duration of ICP elevation per day. These findings should be interpreted cautiously, as most included studies were limited by small sample sizes and insufficient data for certain parameters. Further large-scale, well-designed trials are essential to validate these observations and enhance confidence in the effect estimates. Importantly, given that mannitol is a naturally occurring sugar alcohol derived from plants, this study underscores how phytochemicals can transition into critical clinical applications, thereby reinforcing the bridge between plant-based pharmaceutical research and neurocritical care.
Author Contributions
Conceptualization: Mohamed S. Imam; Methodology: Mohamed S. Imam and Rahaf Naif Alhashbari; Software: Mohamed S. Imam; Validation: Rahaf Naif Alhashbari and Amal Ali Mohammed Alshehri; Formal analysis: Amal Ali Mohammed Alshehri and Shoog Hameed Mohammad Altalhi; Investigation: Mohamed S. Imam; Resources: Atheer Zayed Safar Alotaibi and Nardeen Awad Saed Algethami; Data curation: Shoog Hameed Mohammad Altalhi; Writing-original draft preparation: Mohamed S. Imam, Rahaf Naif Alhashbari, Randa Matuq Aljuaid, Wejdan Muhsen Alsufyane, Ibtisam Eidhah Althubyani; Writing-review and editing: Mohamed S. Imam, Abdullah Mahammed Jaber Almalki, Faisal Saleh Saeed Alswat, Atheer Zayed Safar Alotaibi, Nardeen Awad Saed Algethami, Shoog Hameed Mohammad Altalhi, Amal Ali Mohammed Alshehri; Visualization: Mohamed S. Imam; Supervision: Mohamed S. Imam; Project administration: Mohamed S. Imam; Funding acquisition: Mohamed S. Imam. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Graham DI, Adams JH, Doyle D. (1978). Ischaemic brain damage in fatal non-missile head injuries. J Neurol Sci. 39:213-34.
[Crossref] [Google Scholar] [PubMed]
- Marion DW, Darby J, Yonas H. (1991). Acute regional cerebral blood flow changes caused by severe head injuries. J Neurosurg. 74:407-14.
[Crossref] [Google Scholar] [PubMed]
- Bouma GJ, Muizelaar JP, Choi SC, Newlon PG, Young HF. (1991). Cerebral circulation and metabolism after severe traumatic brain injury: the elusive role of ischemia. J Neurosurg. 75:685-93.
[Crossref] [Google Scholar] [PubMed]
- Katayama Y, Tsubokawa T, Miyazaki S, Kawamata T, Yoshino A. (1990). Oedema fluid formation within contused brain tissue as a cause of medically uncontrollable elevation of intracranial pressure: the role of surgical therapy. Acta Neurochir Suppl (Wien). 51:308-10.
[Crossref] [Google Scholar] [PubMed]
- Scalfani MT, Dhar R, Zazulia AR, Videen TO, Diringer MN. (2012). Effect of osmotic agents on regional cerebral blood flow in traumatic brain injury. J Crit Care. 27:526-e7.
[Crossref] [Google Scholar] [PubMed]
- Firsching R, Rickels E, Mauer UM, Sakowitz OW, Messing-Juenger M, Engelhard K, Schwenkreis P, Linn J, Schwerdtfeger K. (2017). Guidelines for the treatment of head injury in adults. J Neurol Surg A Cent Eur Neurosurg. 78:478-87.
[Crossref] [Google Scholar] [PubMed]
- Carney N, Totten AM, O'Reilly C, Ullman JS, Hawryluk GW, Bell MJ, Bratton SL, Chesnut R, Harris OA, Kissoon N, Rubiano AM. (2017). Guidelines for the management of severe traumatic brain injury. Neurosurgery. 80:6-15.
[Crossref] [Google Scholar] [PubMed]
- Kochanek PM, Tasker RC, Carney N, Totten AM, Adelson PD, Selden NR, Davis-O’Reilly C, Hart EL, Bell MJ, Bratton SL, Grant GA. (2019). Guidelines for the management of pediatric severe traumatic brain injury: Update of the brain trauma foundation guidelines. Pediatr Crit Care Med. 20(3S):S1-82.
[Crossref] [Google Scholar] (All versions) [PubMed]
- Chen TH, Murata N. (2002). Enhancement of tolerance of abiotic stress by metabolic engineering of betaines and other compatible solutes. Curr Opin Plant Biol. 5:250-7.
[Crossref] [Google Scholar] [PubMed]
- Loescher WH. (1987). Physiology and metabolism of sugar alcohols in higher plants. Physiol Plant. 70:553.
- Zhifang G, Loescher WH. (2003). Expression of a celery mannose 6âphosphate reductase in Arabidopsis thaliana enhances salt tolerance and induces biosynthesis of both mannitol and a glucosylâmannitol dimer. Plant Cell Environ. 26:275-83.
- Saha BC, Racine FM. (2011). Biotechnological production of mannitol and its applications. Appl Microbiol Biotechnol. 89:879-91.
[Crossref] [Google Scholar] [PubMed]
- Meena M, Prasad V, Zehra A, Gupta VK, Upadhyay RS. (2015). Mannitol metabolism during pathogenic fungal–host interactions under stressed conditions. Front Microbiol. 6:1019.
[Crossref] [Google Scholar] [PubMed]
- Zhang W, Neal J, Lin L, Dai F, Hersey DP, McDonagh DL, Su F, Meng L. (2019). Mannitol in critical care and surgery over 50+ years: a systematic review of randomized controlled trials and complications with meta-analysis. J Neurosurg Anesthesiol. 31:273-84.
[Crossref] [Google Scholar] [PubMed]
- Shi J, Tan L, Ye J, Hu L. (2020). Hypertonic saline and mannitol in patients with traumatic brain injury: A systematic and meta-analysis. Medicine. 99:e21655.
[Crossref] [Google Scholar] [PubMed]
- Gu J, Huang H, Huang Y, Sun H, Xu H. (2019). Hypertonic saline or mannitol for treating elevated intracranial pressure in traumatic brain injury: A meta-analysis of randomized controlled trials. Neurosurg Rev. 42:499-509.
[Crossref] [Google Scholar] [PubMed]
- Schwimmbeck F, Voellger B, Chappell D, Eberhart L. (2021). Hypertonic saline versus mannitol for traumatic brain injury: A systematic review and meta-analysis with trial sequential analysis. J Neurosurg Anesthesiol. 33:10-20.
[Crossref] [Google Scholar] [PubMed]
- Soustiel JF, Vlodavsky E, Zaaroor M. (2006). Relative effects of mannitol and hypertonic saline on calpain activity, apoptosis and polymorphonuclear infiltration in traumatic focal brain injury. Brain Res. 1101:136-44.
[Crossref] [Google Scholar] [PubMed]
- Anestesiologica MI. (2015). Treatment of intra-parenchymal hypertension with hyperosmotic therapy: Hypertonic saline 7.45% vs. mannitol 20%. Minerva Anestesiol.
- Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, Moher D, Becker BJ, Sipe TA, Thacker SB. (2000). Meta-analysis of observational studies in epidemiology: A proposal for reporting. JAMA. 283:2008-12.
[Crossref] [Google Scholar] [PubMed]
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. (2003). Measuring inconsistency in meta-analyses. BMJ. 327:557-60.
[Crossref] [Google Scholar] [PubMed]
- Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, Clarke M, Devereaux PJ, Kleijnen J, Moher D. (2009). The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: Explanation and elaboration. J Clin Epidemiol. 62:e1-34.
[Crossref] [Google Scholar] [PubMed]
- Gupta A, Das A, Majumder K, Arora N, Mayo HG, Singh PP, Beg MS, Singh S. (2018). Obesity is independently associated with increased risk of hepatocellular cancer–related mortality: A systematic review and meta-analysis. Am J Clin Oncol. 41:874-81.
[Crossref] [Google Scholar] [PubMed]
- RoB C. (2020). 2: A revised Cochrane risk-of-bias tool for randomized trials.
- Vialet R, Albanèse J, Thomachot L, Antonini F, Bourgouin A, Alliez B, Martin C. (2003). Isovolume hypertonic solutes (sodium chloride or mannitol) in the treatment of refractory posttraumatic intracranial hypertension: 2 mL/kg 7.5% saline is more effective than 2 mL/kg 20% mannitol. Crit Care Med. 31:1683-7.
[Crossref] [Google Scholar] [PubMed]
- Harutjunyan L, Holz C, Rieger A, Menzel M, Grond S, Soukup J. (2005). Efficiency of 7.2% hypertonic saline hydroxyethyl starch 200/0.5 versus mannitol 15% in the treatment of increased intracranial pressure in neurosurgical patients–a randomized clinical trial [ISRCTN62699180]. Critical Care. 9:R530.
- Mao X, Feng D, Ye F. (2007). Comparison of mannitol with hypertonic saline in treatment of traumatic brain edema associated with intracranial hypertension. Jiangsu Med J. 33:452.
- Francony G, Fauvage B, Falcon D, Canet C, Dilou H, Lavagne P, Jacquot C, Payen JF. (2008). Equimolar doses of mannitol and hypertonic saline in the treatment of increased intracranial pressure. Crit Care Med. 36:795-800.
[Crossref] [Google Scholar] [PubMed]
- Oddo M, Levine JM, Frangos S, Carrera E, Maloney-Wilensky E, Pascual JL, Kofke WA, Mayer SA, LeRoux PD. (2009). Effect of mannitol and hypertonic saline on cerebral oxygenation in patients with severe traumatic brain injury and refractory intracranial hypertension. J Neurol Neurosurg Psychiatry. 80:916-20.
[Crossref] [Google Scholar] [PubMed]
- Kerwin AJ, Schinco MA, Tepas III JJ, Renfro WH, Vitarbo EA, Muehlberger M. (2009). The use of 23.4% hypertonic saline for the management of elevated intracranial pressure in patients with severe traumatic brain injury: A pilot study. J Trauma. 67:277-282.
[Crossref] [Google Scholar] [PubMed]
- Ichai C, Armando G, Orban JC, Berthier F, Rami L, Samat-Long C, Grimaud D, Leverve X. (2009). Sodium lactate versus mannitol in the treatment of intracranial hypertensive episodes in severe traumatic brain-injured patients. Intensive Care Med. 35:471-9.
[Crossref] [Google Scholar] [PubMed]
- Cottenceau V, Masson F, Mahamid E, Petit L, Shik V, Sztark F, Zaaroor M, Soustiel JF. (2011). Comparison of effects of equiosmolar doses of mannitol and hypertonic saline on cerebral blood flow and metabolism in traumatic brain injury. J Neurotrauma. 28:2003-12.
[Crossref] [Google Scholar] [PubMed]
- Sakellaridis N, Pavlou E, Karatzas S, Chroni D, Vlachos K, Chatzopoulos K, Dimopoulou E, Kelesis C, Karaouli V. (2011). Comparison of mannitol and hypertonic saline in the treatment of severe brain injuries. J Neurosurg. 114:545-8.
[Crossref] [Google Scholar] [PubMed]
- Hendoui N, Beigmohammadi MT, Mahmoodpoor A, Ahmadi A, Abdollahi M, Hasanpour M, Hadi F, Khazaeipour Z, Mousavi S, Mojtahedzadeh M. (2013). Reliability of calcium-binding protein S100B measurement toward optimization of hyperosmolal therapy in traumatic brain injury. Eur Rev Med Pharmacol Sci. 17:477-85.
[Google Scholar] [PubMed]
- Huang X, Yang L. (2014). Comparison of 20% mannitol and 15% hypertonic saline in doses of similar osmotic burden for treatment of severe traumatic brain injury with intracranial hypertension. 34:723-6.
[Google Scholar] [PubMed]
- Jagannatha AT, Sriganesh K, Devi BI, Rao GS. (2016). An equiosmolar study on early intracranial physiology and long term outcome in severe traumatic brain injury comparing mannitol and hypertonic saline. J Clin Neurosci. 27:68-73.
[Crossref] [Google Scholar] [PubMed]
- Du DY, Sun LT, Zhang WS, Li K, Xu C, Li ZF. (2017). The clinical efficacy of hypertonic saline in reducing intracranial pressure in patients with severe traumatic brain injury. NNR. 12:215-17.
- Qin D, Hunag W, Yang L, Huang C, Liang Y, Luo Y, Huang S, Zhu S. (2018). Hypertonic saline in treatment of intracranial hypertension caused by severe cerebral trauma after decompressive craniectomy. Chin J Neuromed. 15:1267-73.
- Patil H, Gupta R. (2019). A comparative study of bolus dose of hypertonic saline, mannitol, and mannitol plus glycerol combination in patients with severe traumatic brain injury. World Neurosurg. 125:e221-8.
[Crossref] [Google Scholar] [PubMed]
- Huang X, Yang L, Ye J, He S, Wang B. (2020). Equimolar doses of hypertonic agents (saline or mannitol) in the treatment of intracranial hypertension after severe traumatic brain injury. Medicine. 99:e22004.
[Crossref] [Google Scholar] [PubMed]
- Mangat HS, Wu X, Gerber LM, Schwarz JT, Fakhar M, Murthy SB, Stieg PE, Ghajar J, Härtl R. (2020). Hypertonic saline is superior to mannitol for the combined effect on intracranial pressure and cerebral perfusion pressure burdens in patients with severe traumatic brain injury. Neurosurgery. 86:221-30.
[Crossref] [Google Scholar] [PubMed]
- Van Veen E, Nieboer D, Kompanje EJ, Citerio G, Stocchetti N, Gommers D, Menon DK, Ercole A, Maas AI, Lingsma HF, Van Der Jagt M. (2023). Comparative effectiveness of mannitol versus hypertonic saline in patients with traumatic brain injury: A CENTER-TBI study. J Neurotrauma. 40:1352-65.
[Crossref] [Google Scholar] [PubMed]
- Maas AI, Menon DK, Adelson PD, Andelic N, Bell MJ, Belli A, Bragge P, Brazinova A, Büki A, Chesnut RM, Citerio G. (2017). Traumatic brain injury: integrated approaches to improve prevention, clinical care, and research. Lancet. 16:987-1048.
[Crossref] [Google Scholar] [PubMed]
- Juul N, Morris GF, Marshall SB, Marshall LF. (2001). Intracranial hypertension and cerebral perfusion pressure: Influence on neurological deterioration and outcome in severe head injury. J Neurosurg. 11:1-6.
[Crossref] [Google Scholar] [PubMed]
- Rangel-Castillo L, Gopinath S, Robertson CS. (2008). Management of intracranial hypertension. Neurol Clin. 26:521-41.
[Crossref] [Google Scholar] [PubMed]
- Gonda DD, Meltzer HS, Crawford JR, Hilfiker ML, Shellington DK, Peterson BM, Levy ML. (2013). Complications associated with prolonged hypertonic saline therapy in children with elevated intracranial pressure. Pediatr Crit Care Med. 14:610-20.
[Crossref] [Google Scholar] [PubMed]
- Treib J, Haass A, Pindur G. (1997). Coagulation disorders caused by hydroxyethyl starch. Thromb Haemost. 78:0974-83.
[Crossref] [Google Scholar] [PubMed]
- Moon PF, Kramer GC. (1995). Hypertonic saline-dextran resuscitation from hemorrhagic shock induces transient mixed acidosis. Crit Care Med. 23:323-31.
[Crossref] [Google Scholar] [PubMed]
- Kempski O, Obert C, Mainka T, Heimann A, Strecker U. (1996). “Small volume resuscitation” as treatment of cerebral blood flow disturbances and increased ICP in trauma and ischemia. Acta Neurochir Suppl. 66:114-7.
[Crossref] [Google Scholar] [PubMed]
- Battison C, Andrews PJ, Graham C, Petty T. (2005). Randomized, controlled trial on the effect of a 20% mannitol solution and a 7.5% saline/6% dextran solution on increased intracranial pressure after brain injury. Crit Care Med. 33:196-202.
[Crossref] [Google Scholar] [PubMed]